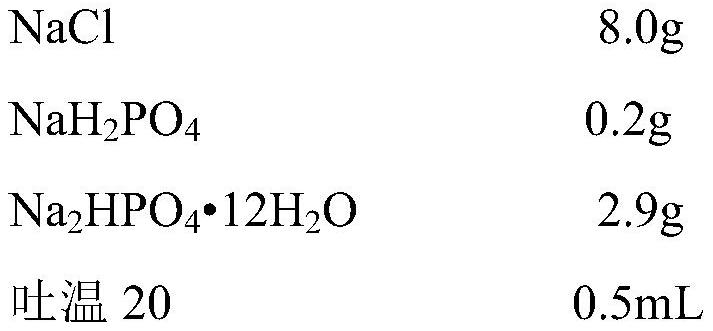Novel coronavirus neutralizing antibody detection kit based on magnetic particle chemiluminescence and application thereof
A chemiluminescence and antibody detection technology, applied in the field of clinical testing, to achieve the effect of easy operation, low cost and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Preparation of a neogular virus and antibody detection tester according to a magnetic particulate chemiluminescence of the present invention
[0073] (1) Preparation of magnetic microspheres:
[0074] 1.1) Formulate the coupling buffer: i.e. to formulate 0.01 mol / L MES buffer solution, and adjust the pH of 6.0;
[0075] MES 1.95G
[0076] Purification water to 1000ml
[0077] Filtration and sterilization, stored at 4 ° C;
[0078] 1.2) The formulation and method for preparing the washing liquid, the washing liquid is:
[0079]
[0080] 1.3) Formulation of preservation fluids, preservation fluids is:
[0081]
[0082] 1.4) Coupling operation
[0083] (1) RBD protein 1 acidification treatment
[0084] Acid treatment: Take 1 mL of concentration of 1 mg / ml of RBD protein 1 solution to 2.0 ml of low adsorption EP tube, add 0.2 ml of acidification treatment liquid a to the EP tube, and mix well at room temperature 40 rpm.
[0085] Metallization: 0.4 ml of treat...
Embodiment 2
[0117] Example 2: The use of magneto-particulate chemiluminescent and antibody detection kits based on magneto-particulate chemiluminescence and antibody detection kits in detecting new types of coronary viruses and antibody biological samples.
[0118] Among them, the preferred method of detecting a new type of coronavirus and antibody sample is:
[0119] a) Add 50 μl of sample 5 μl, 50 μl of sample dilution, and add concentration of 0.2-0.5 mg / ml to 50 μl of a concentration of 0.2-0.5 mg / ml to the reaction cup. Positive control, negative control Press a) the same step operation;
[0120] b) incubation for 15 minutes at 37 ° C after mixing;
[0121] c) Wash 4-5 times with magnetic separation frame or magnetic separator.
[0122] d) Add a horseradish peroxidase-labeled recombinant RBD protein 2 solution 100 uL to the fully automatic magnetic particle chemiluminescence analyzer reaction cup;
[0123] e) incubate at 37 ° C for 15 minutes after mixing;
[0124] f) Wash 4-5 times ...
Embodiment 3
[0132] Example 3: Detection of linearity, accuracy, and precision detection of a neogignic micro crown virus and antibody detection kit of a neogignic micro-crown and antibody detection kit
[0133] 1) Linearity of the kit
[0134] (1) The subscript formulation: RBD neutralizing antibody was diluted to 10 ng / ml, 20 ng / ml, 50 ng / ml, 100 ng / ml, 250 ng / ml, 500 ng / ml, 1000 ng / ml.
[0135] (2) Test: The method of formulation is detected by the method described in Example 2 to obtain an RLU value;
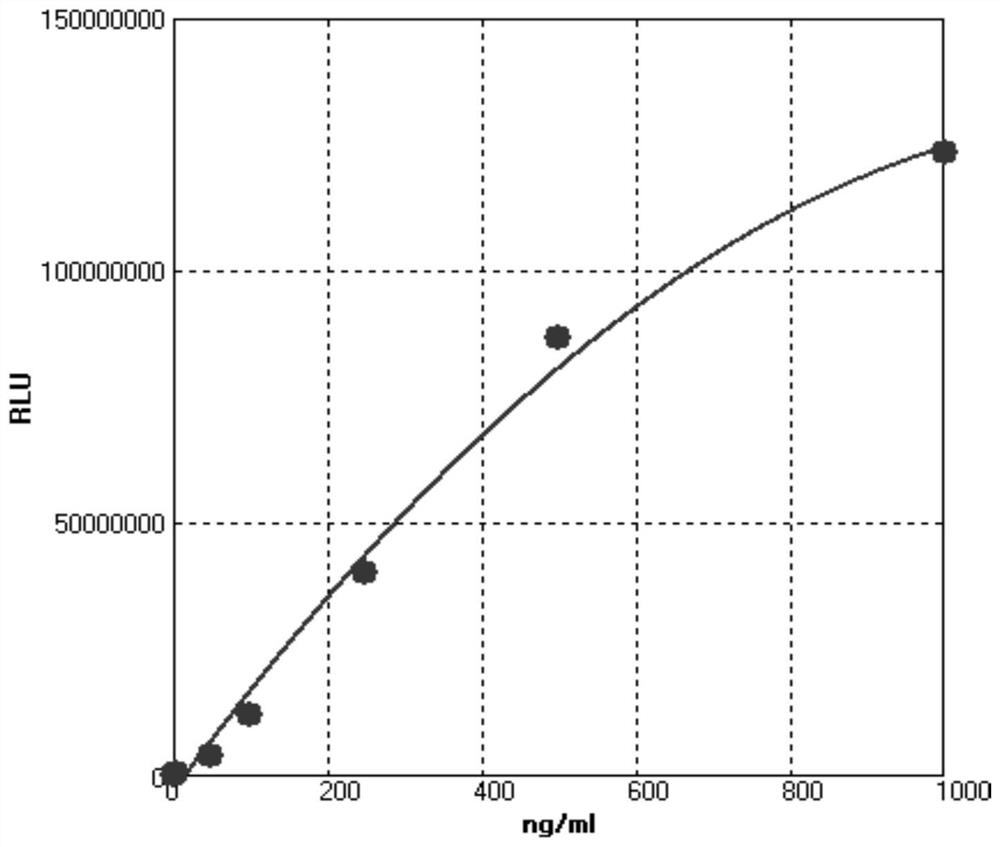
[0136] (3) Establishment of standard curve: The calibration curve of the reagent is generated according to the preparation concentration of the calibration and the RLU value employ a suitable fitting manner. See Table 1 and figure 1 .
[0137] Table 1 kit linearity
[0138] concentration 0 ng / ml 10 ng / ml 20 ng / ml 50 ng / ml 100 ng / ml 250ng / ml 500ng / ml 1000 ng / ml RLU 23258 398748 736886 4248972 12038794 40245372 86548971 123549786
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com