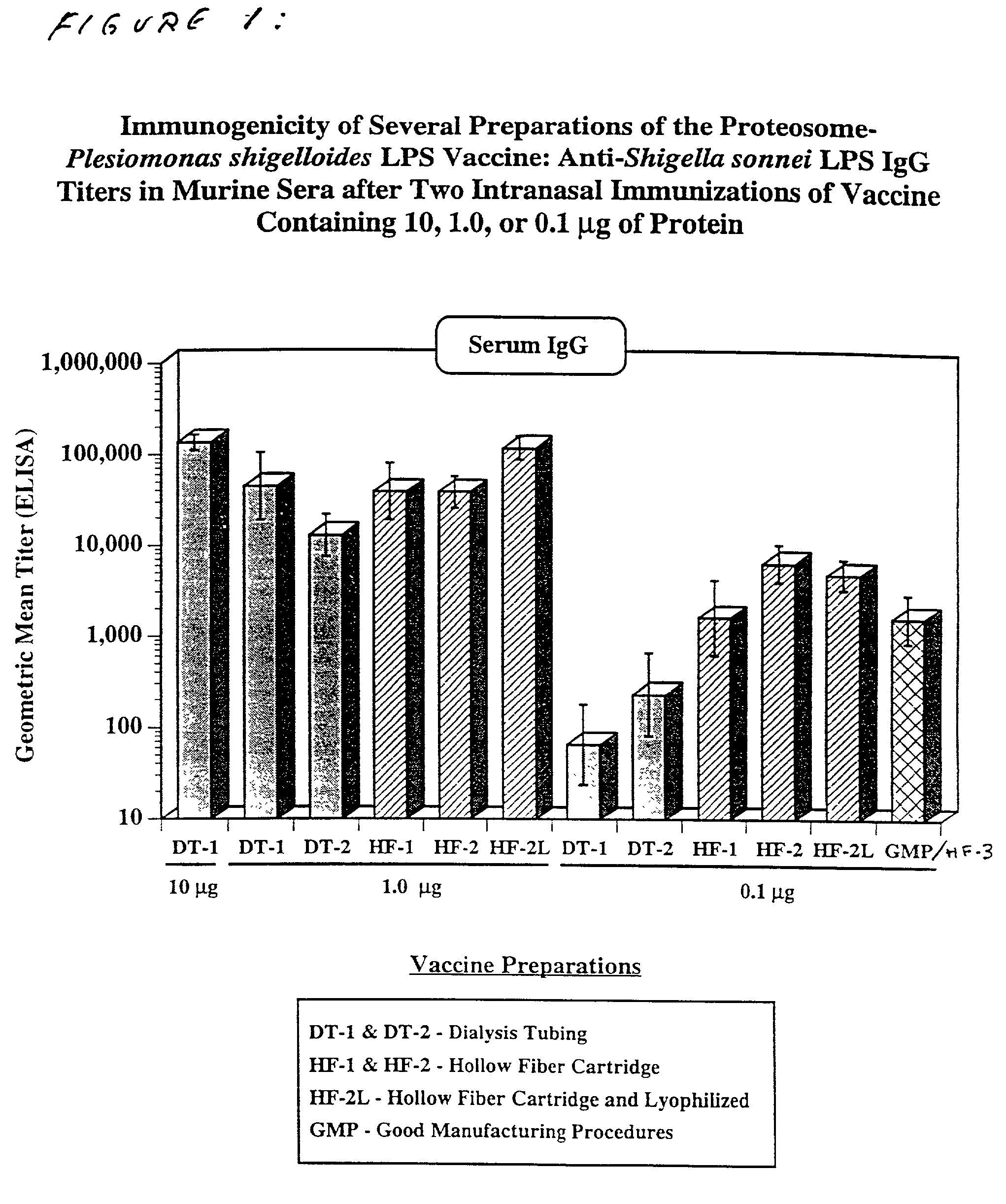
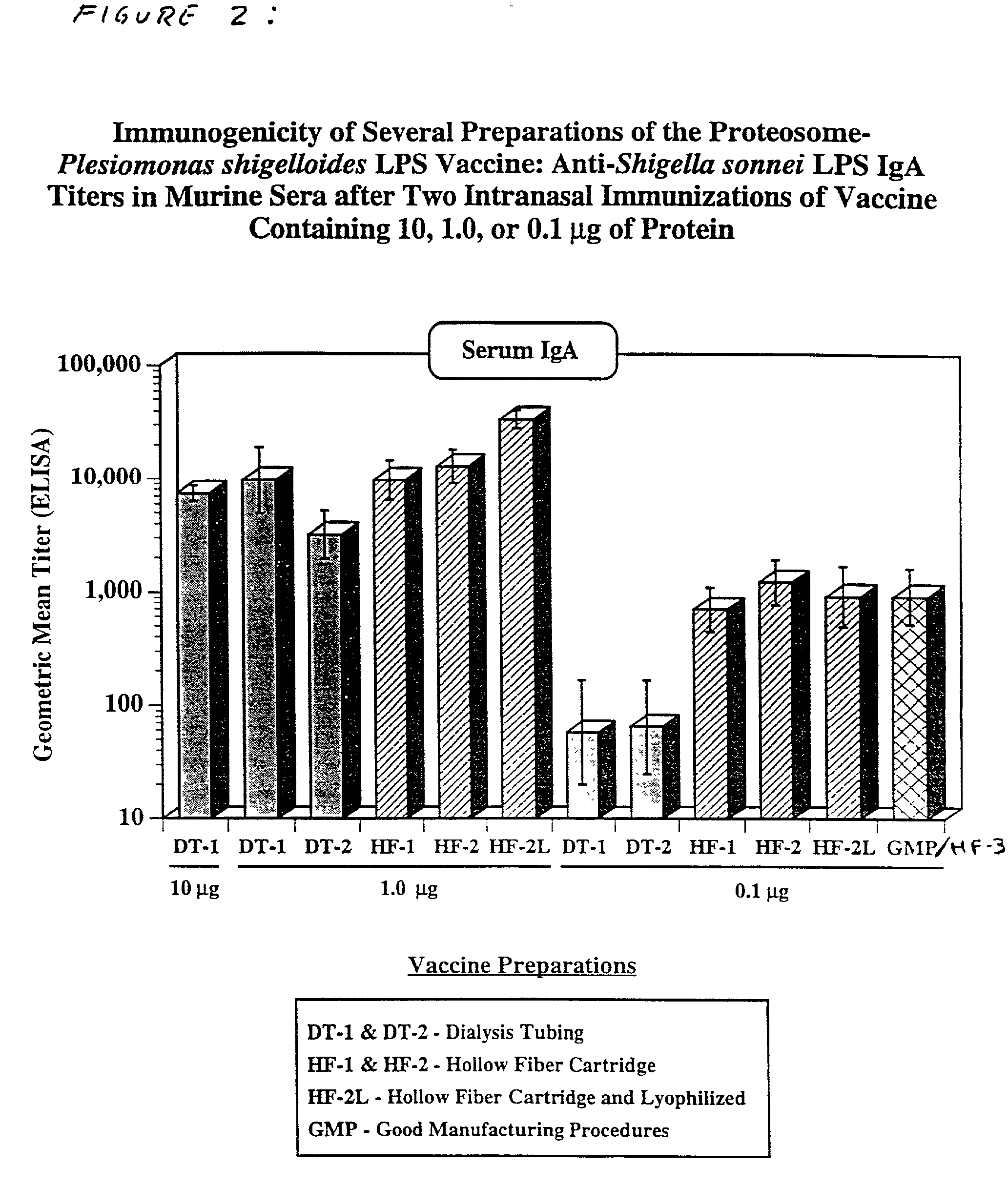
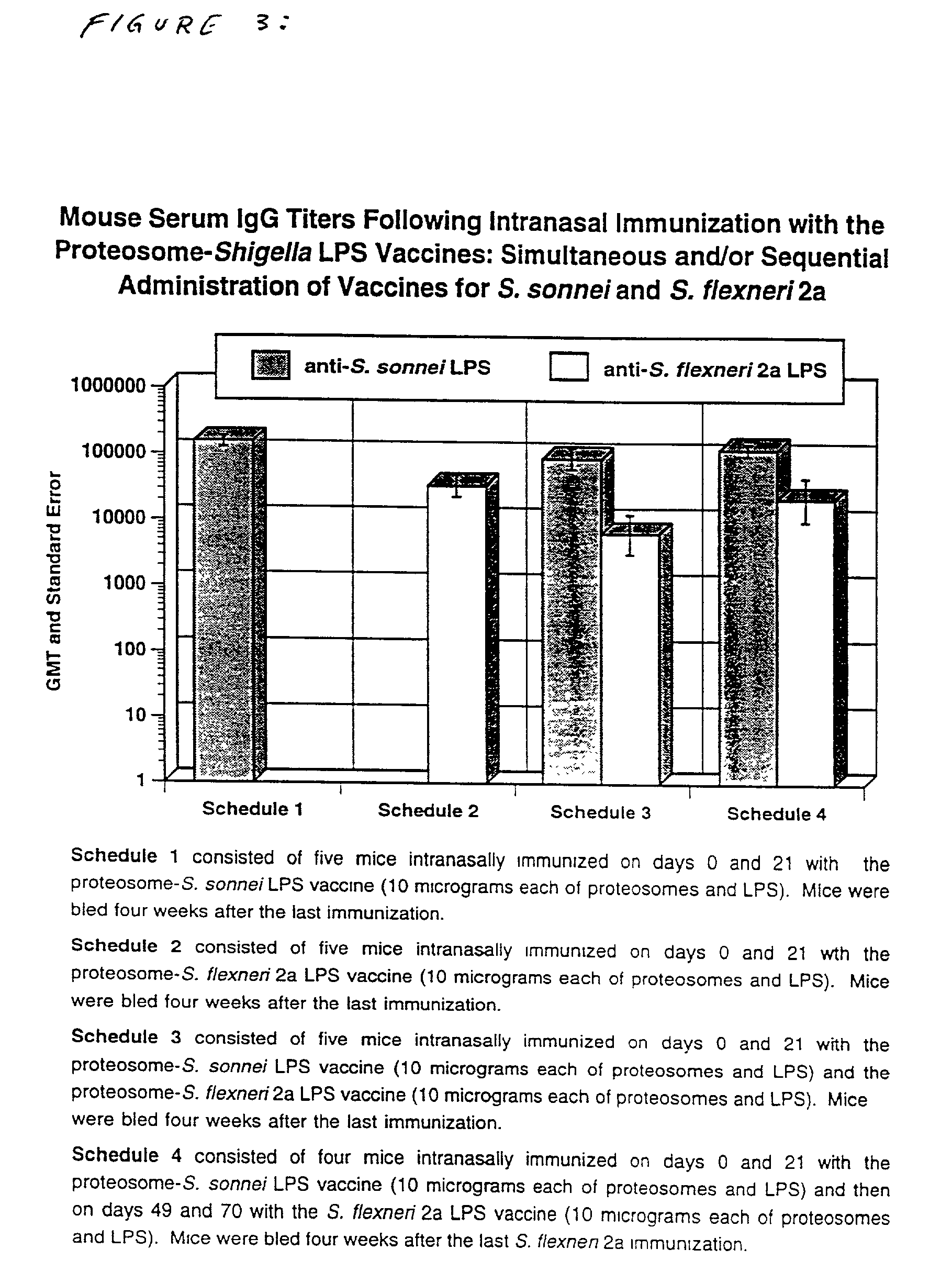
Methods for the production of non-covalently complexed and multivalent proteosome sub-unit vaccines
a technology of proteosomes and vaccines, applied in antibody medical ingredients, carrier-bound antigen/hapten ingredients, immunological disorders, etc., can solve the problems of increasing the opportunity for contamination, preventing the advanced development and commercialization of this technology, and difficult sterilization of tubing, etc., to reduce the chance of contamination, increase the temperature, and reduce the time required
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0043] Noncovalent complexing of bulk Neisselia meningitidis strain 9162 purified outer membrane proteins (proteosomes) and alkaline detoxified N. meningitidis L8 lipooligosaccharide purified from strain 8532.
[0044] 5.1.0 Bulk 9162 outer membrane protein (proteosomes), lot 0136 and bulk detoxified meningococcal L8 Lipooligosaccharide (LOS) lot 0203 were taken from storage and thawed at room temperature. A volume (182 ml) of the bulk LOS containing 500 mg LOS in sterile distilled water was combined with a volume (306 ml) of bulk proteosomes containing 400 mg of protein in buffer containing 0.05 M Tris-HCl, 0.15 M NaCl, 0.01 M EDTA, and 0.1% Empigen BB.
[0045] 5.1.2 Empigen BB (30% solution) was sterile filtered through a 0.22 .mu.m pore size filter and {fraction (1 / 60)}.sup.th volume added to the combined proteosomes, LOS solution and stirred at room temperature for 1 hour.
[0046] 5.1.3 The detergent buffer solution was removed from the proteosomes, LOS solution and replaced with steri...
example 3
[0055] This example is also directed to the production of proteosome-P. shigelloides vaccine. The procedure is equally applicable for scale-up using 10-1000 fold more material with appropriate scale-up of housing size of the membrane cartridge and appropriate increases in the volumes.
[0056] 5.8.1 Preparation of 25 mL Sterile-Filtered Empigen BB (30% Solution)
[0057] 5.8.1.1 Use a 100 ml Nalgene disposable filter unit with 0.2.mu. pore size membrane filter to sterile filter about 25 ml of Empigen BB (30% solution).
[0058] 5.8.2 Prepare 4 L of TEEN 2.times. with 2% Empigen BB (consisting of 0.1 M Tris, 0.02 M disodium EDTA, 0.3 M sodium chloride, WFI and 2% solution of empigen BB).
[0059] 5.8.3 Prepare a 10 L solution of "TNS" fifteen times to total 150 liters: TNS consists of 0.15 M sodium chloride, 0.05 M Tris Buffer pH 8.0.+-.0.2
[0060] 5.8.4 Prepare 10 liters of 0.5 N Sodium Hydroxide
[0061] 5.8.6 If necessary, thaw bulk P. shigelloides lipopolysaccharide (LPS) to prepare for complexin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com