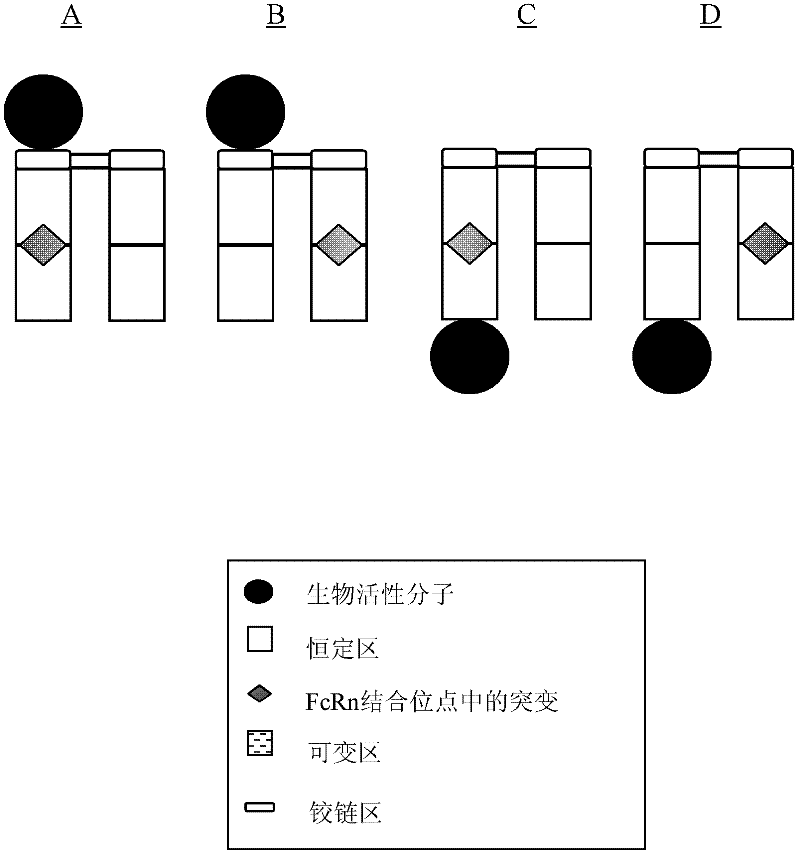
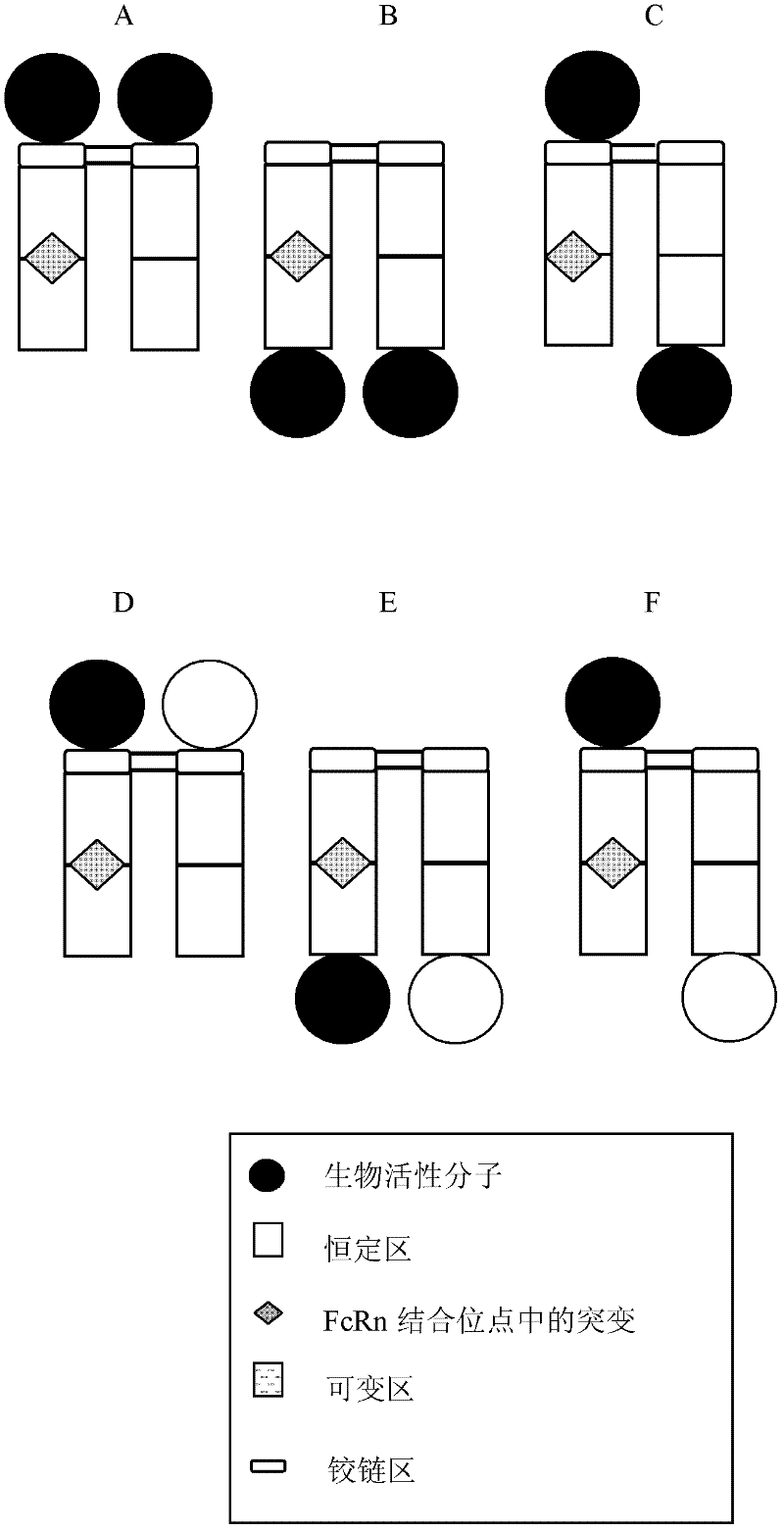
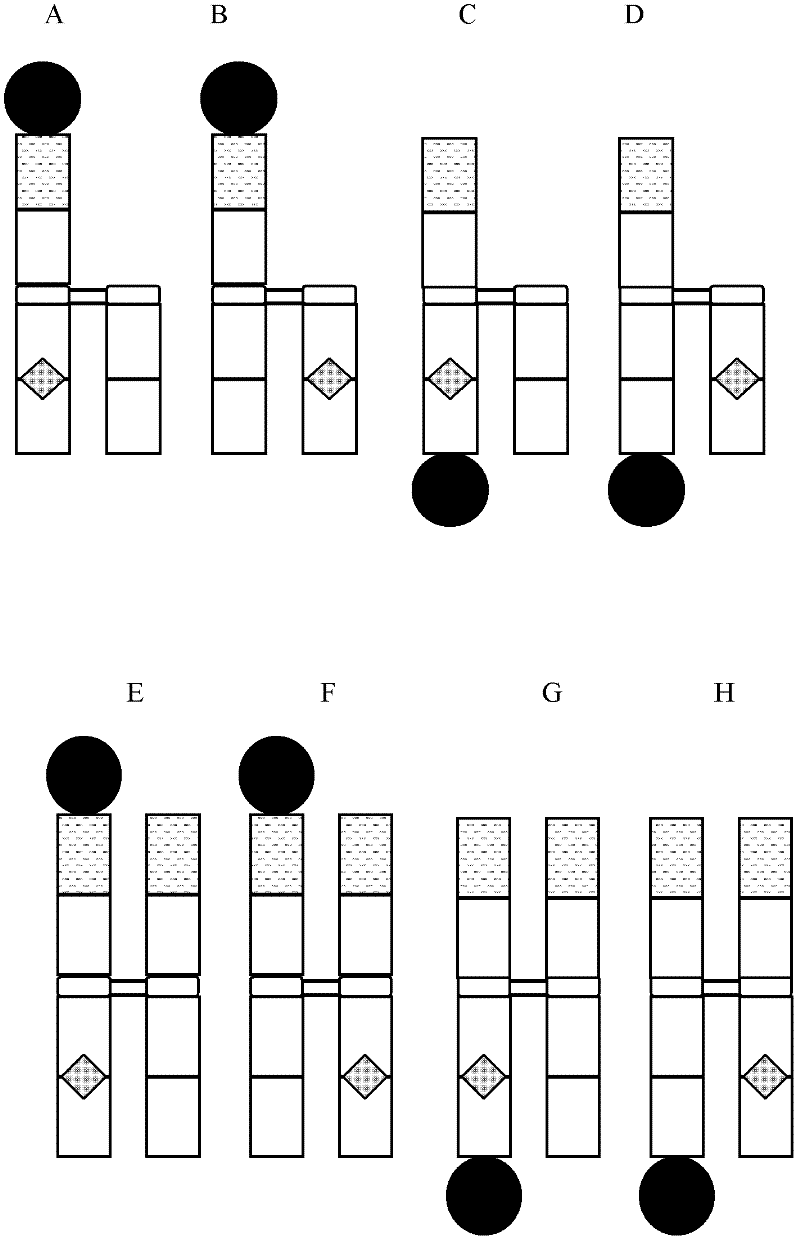
Antibody fusion proteins with modified fcrn binding sites
A technology of fusion protein and antibody, applied in the field of Fc-IFNβ fusion protein, which can solve problems such as reducing serum half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1: Construction of DNA sequence for expressing huFcV4h-single-L-DI-IFNβ
[0114] The huFcγ4h-mono-L-DI-IFNβ (huFcγ4 hinge mutant-linker monomer deimmunized interferon-β) antibody fusion protein is a heterologous human Fcγ4h chain and human Fc-γ4h-linker-DI-IFNβ chain dimer. The protein was produced in mammalian cells by co-expressing human Fcγ4h chain and human Fc-γ4h-linker-DI-IFNβ chain with its transcription unit contained in a single plasmid or 2 separate plasmids.
[0115] DNA encoding huFcγ4h (huFcγ4 hinge mutant) was derived from human IgG4 genomic sequence and subsequently engineered to contain a modified γ1 hinge region in order to minimize half-molecule formation. The formation of IgG4 half-molecules that do not form covalent disulfide bonds at the hinge region has been previously reported (Angal et al. (1993) M0l. Immunol. 30:105).
[0116] Construction of DNA Sequence Encoding Fc Fragment of Human Immunoglobulin-γ4 (Fcγ4)
[0117] The genomic ...
Embodiment 2
[0141] Example 2: Construction for expression of huFcv4h-single - DNA sequence of the L-DI-IFNβ variant
[0142] The DNA sequence for expression of the huFcv4h-mono-L-DI-IFNβ variant containing the H435A substitution (Kabat numbering) in γ4 was constructed. This includes genes encoding the heterodimers huFcγ4h(H435A) / huFcγ4h-L-DI-IFNβ, huFcγ4h / huFcγ4h(H435A)-L-DI-IFNβ and huFcγ4h(H435A) / huFcγ4h(H435A)-L-DI-IFNβ DNA sequence. By overlapping PCR with mutagenic primers (Daugherty et al. (1991) Nucleic Acids Res.19:2471-2476), introducing a mutation from the CAC codon to GCG encoding the H435A substitution in either the naked huFcγ4h chain or the huFcγ4h-L-DI-IFNβ fusion protein chain using the forward primer 5′-GGCTCTGCACAAC GCG TACACGCAGAAGAG (SEQ ID NO: 12), wherein GCG Encoding alanine substitution, and reverse primer 5′-CTCTTCTGCGTGTA CGC GTTGTGCAGAGCC (SEQ ID NO: 13), wherein CGC is the anticodon for alanine substitution.
Embodiment 3
[0143] Embodiment 3: the expression of fusion protein
[0144] The huFcγ4h-mono-L-DI-IFNβ heterodimer was produced in mammalian cells by co-expressing the human Fcγ4h chain and the human Fc-γ4h-linker-DI-IFNβ chain, wherein the transcriptional unit of the chain is contained in 1 single plasmid or 2 separate plasmids. For rapid analysis of protein expression, plasmids pdCs-Fcγ4h and pdCs-Fc Fcγ4h-linker-DI-IFNβ or variants were introduced into human kidney 293T cells by transient transfection using lipofectamine (Invitrogen, Carlsbad, CA) (GenHunter Corporation, Nashville, TN).
[0145] Mouse myeloma NS / 0 and Chinese hamster ovary cells were used to obtain stably transfected clones expressing the huFcγ4h-mono-L-DI-IFNβ heterodimer. For high-level expression, plasmid pdCs containing human Fcγ4h chain and human Fcγ4h-linker-DI-IFNβ chain transcription unit were introduced into mouse myeloma NS / 0 cells by electroporation. NS / 0 cells were grown in Dalbecco's Modified Eagle's M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of freedom | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com