Recombinant antibody vector
A technology of recombinant antibodies and vectors, applied in the fields of biochemical equipment and methods, applications, and botanical equipment and methods, which can solve problems such as limited flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Design and Construction of Recombinant Antibody Vectors
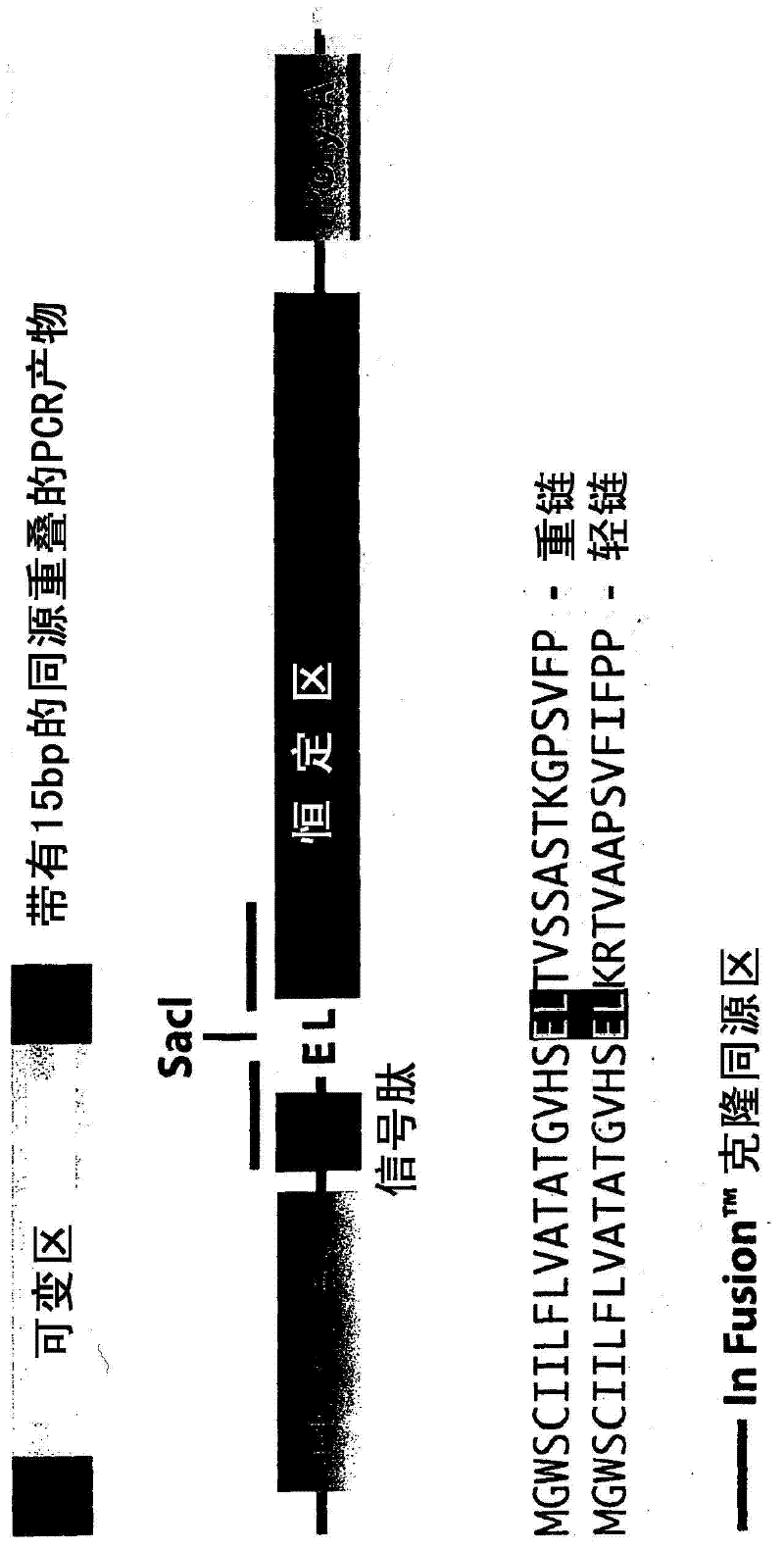
[0094] Ig variable regions were identified to include glutamic acid (E) residues at or near the N-terminus and leucine (L) at or near the C-terminus. This feature appears to occur in about 10% of variable regions. By removing the spacer, a vector can be constructed that encodes the amino acid EL with the nucleotide sequence GAG CTC, thereby forming a SacI site. Insertion of the variable region nucleotide sequence at the SacI site results in a "split" of the EL sequence such that the E residue is N-terminal to the variable region and L is C-terminal to the variable region. This avoids the addition of foreign amino acids, E and L residues are usually found at or near the N- and C-termini of variable regions. A schematic description of the "mAbXpress" recombinant antibody vector is in figure 1 available in .
[0095] method:
[0096] A single restriction site was included after the signal peptide to allow ins...
Embodiment 2
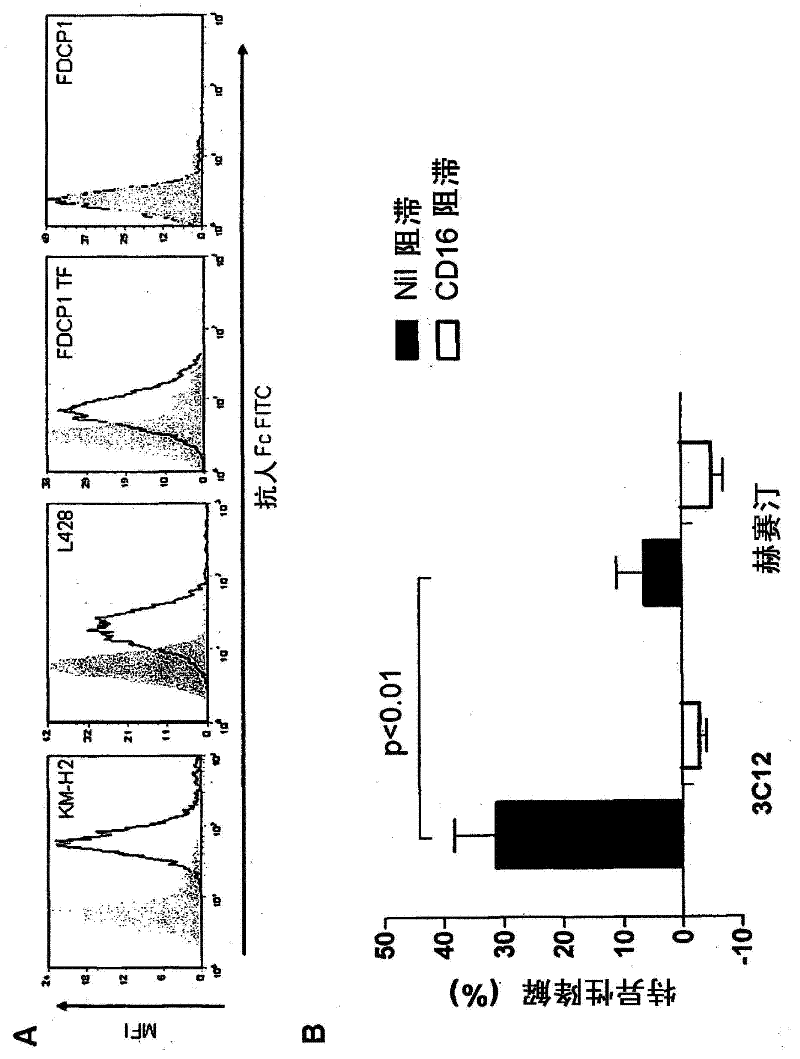
[0199] Production of anti-CD83 recombinant antibody
[0200] 1. Methods and materials:
[0201] 1.1 Design of expression vector.
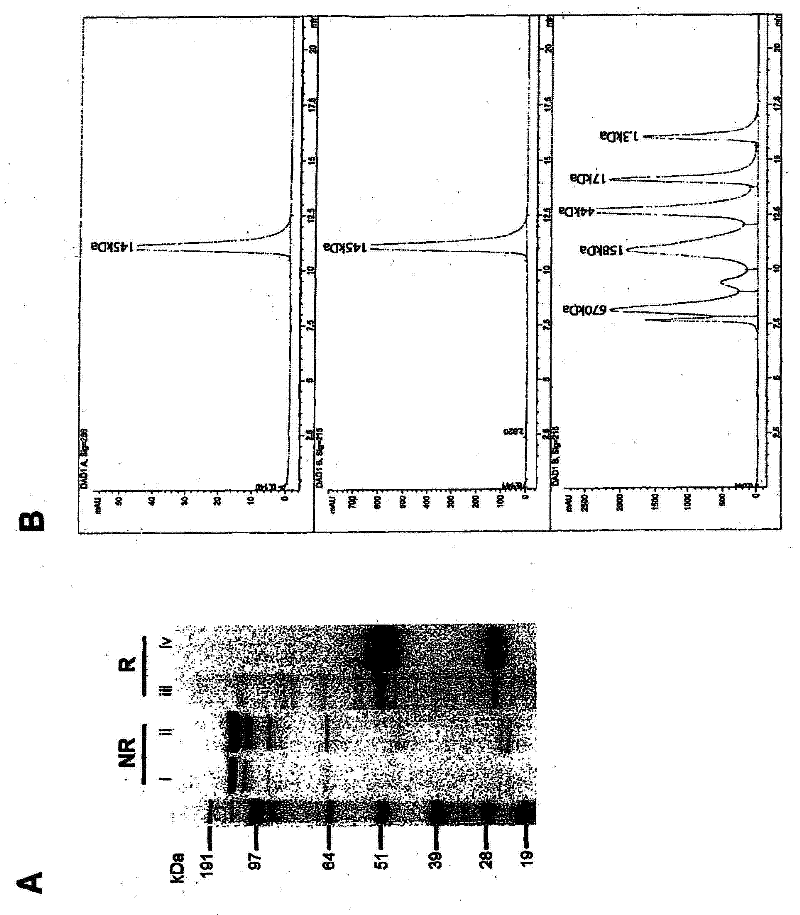
[0202] mAbXpress vectors were assembled as described in Example 1 using publicly available human constant region heavy chain (IgG1 and IgG4 subtypes) and light chain (κ) sequences. The required DNA was synthesized and codon optimized for mammalian expression using Geneart AG (Germany). These cassettes are then placed into mammalian expression vectors containing sequences for expression, selection and amplification in mammalian cells ( figure 1 ). A single SacI site is included in the expression vector to facilitate linearization of variable regions and In Fusion TM Clone (see Section 3 for details).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com