Fusion protein of thymulin alpha1
A technology of fusion protein and thymosin, which is applied in the field of fusion protein of thymosin α1, can solve the problems of short half-life and low immune resistance of tumor sites, and achieve long half-life in vivo and strong anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 Construction of thymosin α1 fusion protein expression vector
[0027] Use the method of gene synthesis to obtain the complementary sequence of the nucleotide sequence corresponding to SEQIDNO: 1 and SEQIDNO: 3. NcoI and HindIII enzyme cutting sites are designed at both ends of the SEQIDNO: 5 sequence, and the 5' of NcoI and HindIII enzyme cutting sites Three guanine protection bases were designed at each end, the complementary sequence was denatured at 94°C for 5 minutes, annealed at 54°C for 10 minutes, then digested with NcoI and HindIII, cloned into the prokaryotic expression vector pET32a, transformed into Escherichia coli, and polymerase chain method Screen for positive transformants. The polymerase chain method primers are:
[0028] P1: 5'-CCATGGCTAGCGATGCGGCCGTGGAT-3' SEQ ID NO: 6
[0029] P2: 5'-AAGCTTTTATTTACCCGGAGACAGGG-3' SEQ ID NO: 7
[0030] The polymerase chain method amplification system is:
[0031] 10 μl each of primer P1 (25 μmol / L) an...
Embodiment 2
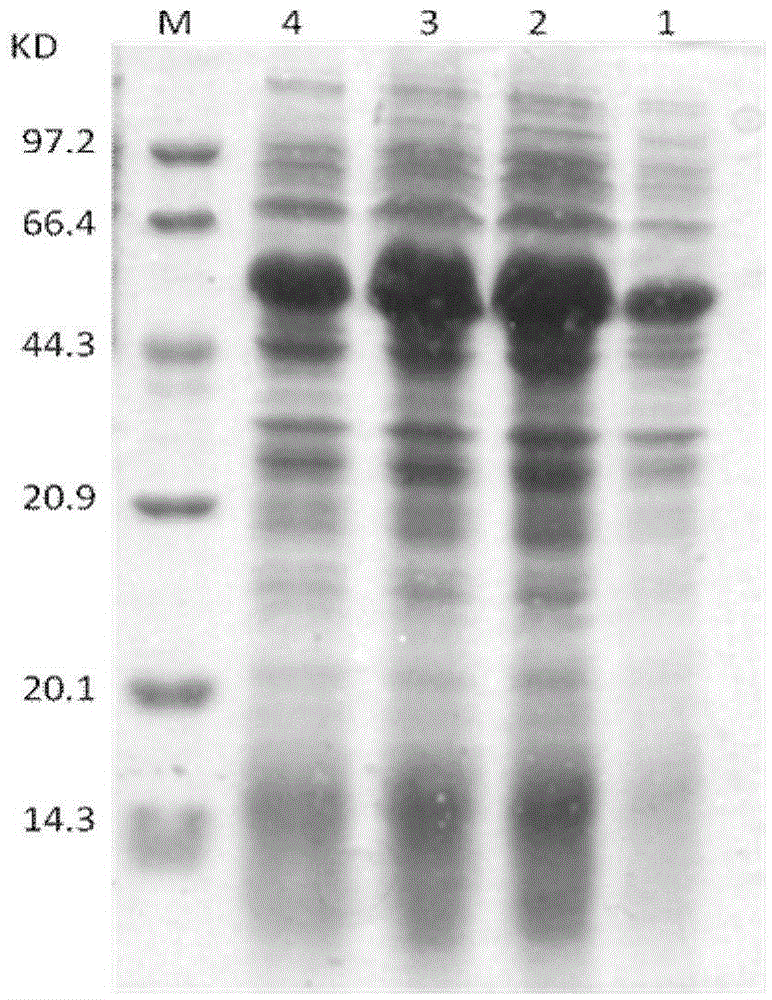
[0033] Induction and separation and purification of the recombinant protein of embodiment 2
[0034] Inoculate the recombinant bacteria from the single bacterium colony on the plate line to 5ml of LB medium, shake overnight, inoculate into 500ml of ampicillin-resistant liquid LB medium with 1% (V / V) inoculation amount, shake at 37 degrees After incubation for 2.5 hours, when OD600 is about 0.6, add 1% (V / V) 0.5mol / L lactose and shake for 1, 2, 3, 4 hours to induce the expression of the target protein, and detect it by electrophoresis, see figure 1 . Centrifuge at 13,000 rpm at 4 degrees for 10 minutes to collect the bacteria, add 20 times the volume of the bacteria in buffer (20mmol / L sodium phosphate at pH7.4, 0.5mol / LNaCl) to suspend the bacteria, and then perform ultrasonication in an ice bath Break up the bacterium, then centrifuge at 13000 rpm at 4 degrees for 10 minutes, draw the supernatant and load it on a metal chelate chromatography column, first use 10 times the co...
Embodiment 3
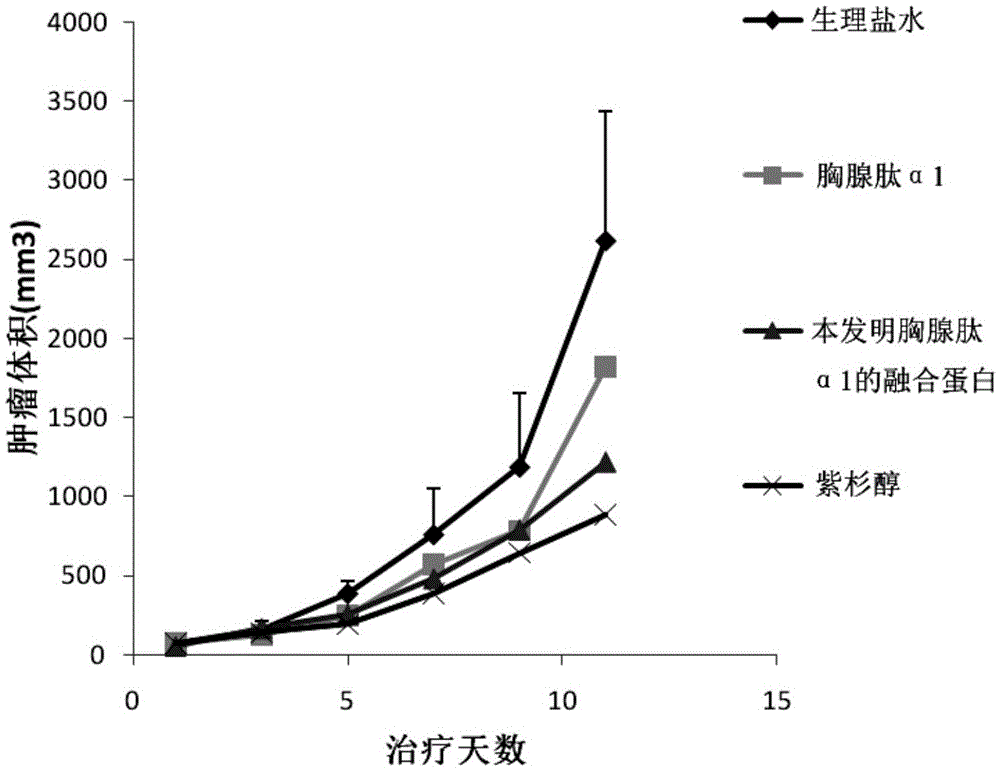
[0035] Example 3 Anti-melanoma Experiment in Animals
[0036] Murine melanoma B16F10 cells were digested with 0.05% trypsin and centrifuged at 1000rpm for 5 minutes, resuspended in PBS, adjusted to 5,000,000 cells per ml, and subcutaneously inoculated on the side of C57BL / 6 (6-8 weeks) mice for about 500,000 cells. When the average tumor volume reaches 100mm 3, randomly divide the mice into 4 groups, 8 mice / group, except the negative control PBS group and the positive control paclitaxel group, one group is given thymosin α1 (dose is 0.25mg / kg / d), and one group is given thymosin α1 fusion protein (The dose is 0.25mg / kg / d, which is in an equimolar relationship with the dose of thymosin α1). kg). The final therapeutic effect is expressed by tumor weight inhibition rate: (1-tumor tumor weight in treatment group / tumor tumor weight in negative control group)×100%
[0037] The result is as figure 2 As shown, when treated to the 11th day, the tumor inhibition rate of thymosin α1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com