Single domain antigen combined molecule purifying method
A binding and molecular technology, applied in the preparation method of peptides, chemical instruments and methods, anti-animal/human immunoglobulin, etc., can solve problems such as obstruction, elution product contamination, product dilution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] Example 1: Description of the ATN-103 coding sequence
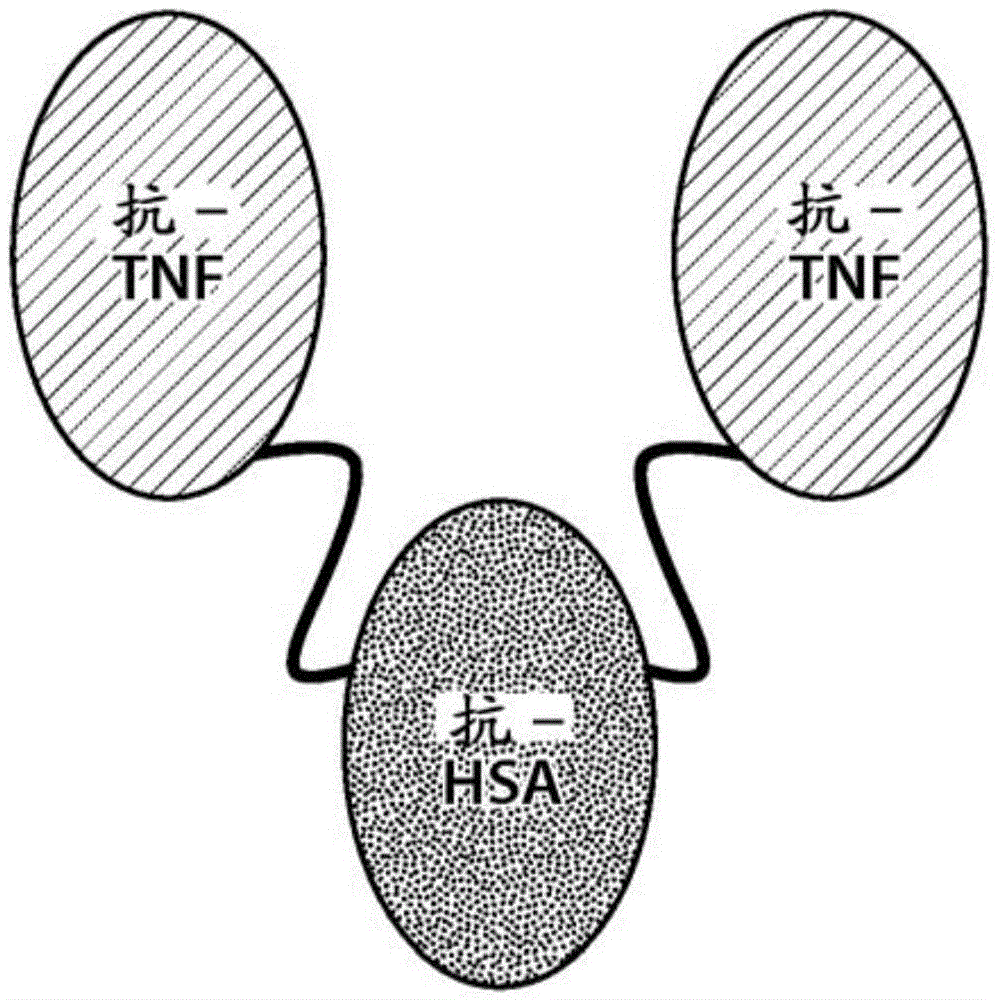
[0191] ATN-103 is a trivalent nanobody molecule targeting TNFα and HSA. Nanobodies were isolated from llama-derived phage libraries by selection for TNFα or HSA as described in WO06 / 122786. The specific activity of the Nanobodies was tested and TNF1 was selected as a Nanobody inhibitor of human TNFα and ALB1 as a human anti-HSA Nanobody for half-life extension. TNF1 and ALB1 were humanized by CDR grafting onto the closest human framework (DP51 / DP53). During the humanization of TNF1, 2 camelid residues (P84 and R103) were retained and this version was named TNF30. During the humanization of ALB1, seven camelid residues (N16, N73, T76, P84, T93, I94 and S103) were retained and this version was named ALB8. Each of the two TNF30 Nanobodies was linked by a 9 amino acid glycine-serine linker (Gly 4 SerGly 3 Ser (SEQ ID NO:9)) was linked to the central ALB8 Nanobody resulting in a trivalent molecule referred to herei...
Embodiment 2
[0192] Embodiment 2: ATN-103 purification process
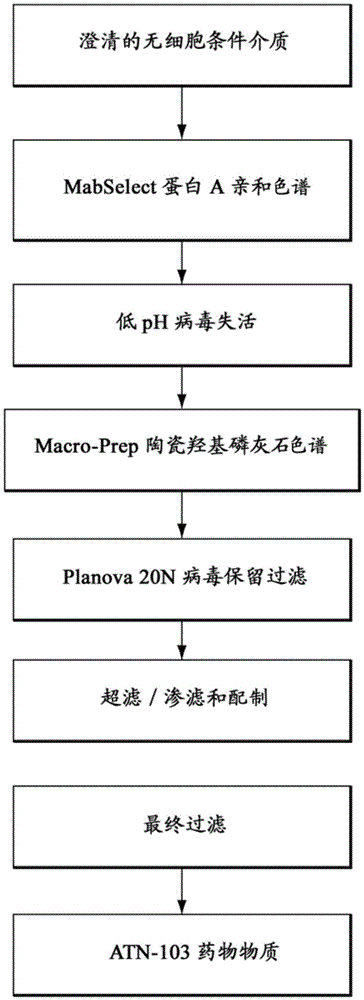
[0193] The ATN-103 purification process consists of two chromatography steps and three membrane filtration steps (see image 3 ). All steps were performed at room temperature unless otherwise noted.
[0194] The rationale, purpose and description of each purification step are provided below.
[0195] MabSelect Protein A affinity chromatography and low pH virus inactivation
[0196] The main purpose of the MabSelect™ Protein A chromatography step includes product capture from the clarified cell-free conditioned media and separation of ATN-103 from process-derived impurities (eg, host cell DNA and proteins, media components and foreign agents).
[0197] MabSelect Protein A is an affinity resin composed of a highly cross-linked agarose matrix covalently derivatized by thioether linkage with recombinant Protein A produced from E. coli fermentation.
[0198] MabSelect Protein A columns were equilibrated with Tris-buffered so...
Embodiment 3
[0212] Example 3: Protein A capture comparison
[0213] The ability of the following Protein A-based matrices to capture ATN-103 was assessed: MabSelect TM (GE Healthcare), MabSelect Xtra TM (GE Healthcare), VaUltraPlus (Millipore) and MabSelectSuRe TM(GE Healthcare). MabSelect TM A protein A ligand containing a Z domain is used, and the resin backbone is more hydrophobic due to cross-linking. MabSelectXtra TM Uses the same ligands as MabSelect with a 30% increase in density, and has smaller beads and larger pore sizes. VaUltraPlus has a glass-based backbone and native protein A ligands. It is designed for higher capacity at higher flow rates. MabSelectSuRe TM Binding of Fc-containing molecules (ATN-103 has no Fc region) and its novel ligand allows for greater caustic stability.
[0214] When using MabSelect TM When the purified protein A peak pool was used as loading material (pH=7.0, diluted to 1g / L (expected condition medium concentration)), VaUltraPlus showe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com