Immunoglobulin constant region fc receptor binding agents
a technology of immunoglobulin and constant region, applied in the field of immunology, inflammation, tumor immunology, can solve the problems of insufficient sterility, lack of availability, presence of impurities, etc., and achieve the effect of broad application, treatment and prophylaxis of pathological conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construct Design of Immunologically Active Biomimetics
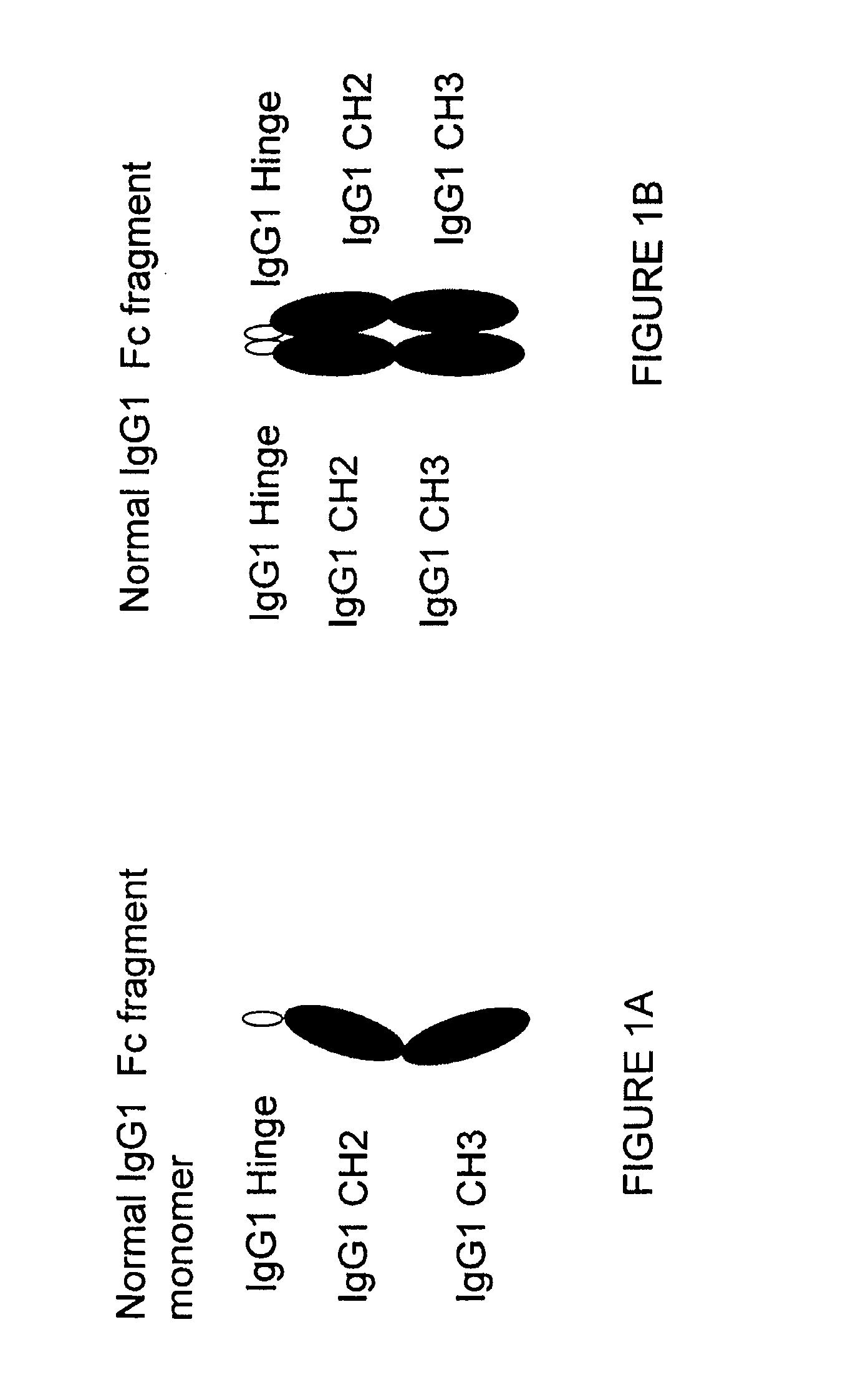
[0345]A sequence encoding a Fc fragment monomer from human IgG1 (SEQ ID NO: 1) has been cloned into an expression vector (pCDNA 3.1D / V5 His TOPO Invitrogen) comprising selected restriction enzyme cleavage sites, an IgK signal (further defined below) and epitope tags to create the IgG1 monomer sequence {RestEnzSites-IgK signal-RestEnzSites-IgG1 (Hinge-CH2-CH3)-RestEnzSites-epitope tags (V5 and His)-STOP), shown in FIG. 17 (SEQ ID NO:19). The construct was transfected into CHO cells (CHO-002) for protein production. Additionally, we have designed several stradomer constructs with the general structures:
[0346](a) {RestEnzSites-IgK signal-RestEnzSites-IgG1(Hinge-CH2-CH3)-XbaI site-IgG1(Hinge-CH2-CH3)-STOP} (SEQ ID NO: 21) (see also FIG. 4A and FIG. 18);
[0347](b) {RestEnzSites-IgK signal-RestEnzSites-IgG1 (Hinge-CH2-CH3)-XbaI site-IgG1 (Hinge-CH2-CH3)-RestEnzSites-epitope tags (V5 and His)-STOP} (SEQ ID NO: 23) (see also FIG. 19);
[034...
example 2
Design and Testing of Immunologically Active Biomimetics Coated IVIG and Coated Fc stimulate Similar Phenotypic Changes
[0354]IVIG and Fc when coated onto the walls and floor of the wells of a sterile plate stimulate nearly identical changes in CD1a and CD86 levels on immature DC and delay the up regulation of CD11c. Because of the recognized critical role of DC in ITP, these data provide a rational model for evaluating the function of IVG mimetics such as stradomers. We also conclude that the fact that the phenotypic changes induced by IVIG are completely recapitulated by recombinant Fc suggest that the effects of IVIG on DC are highly likely to be Fc mediated.
Stradomer Generation
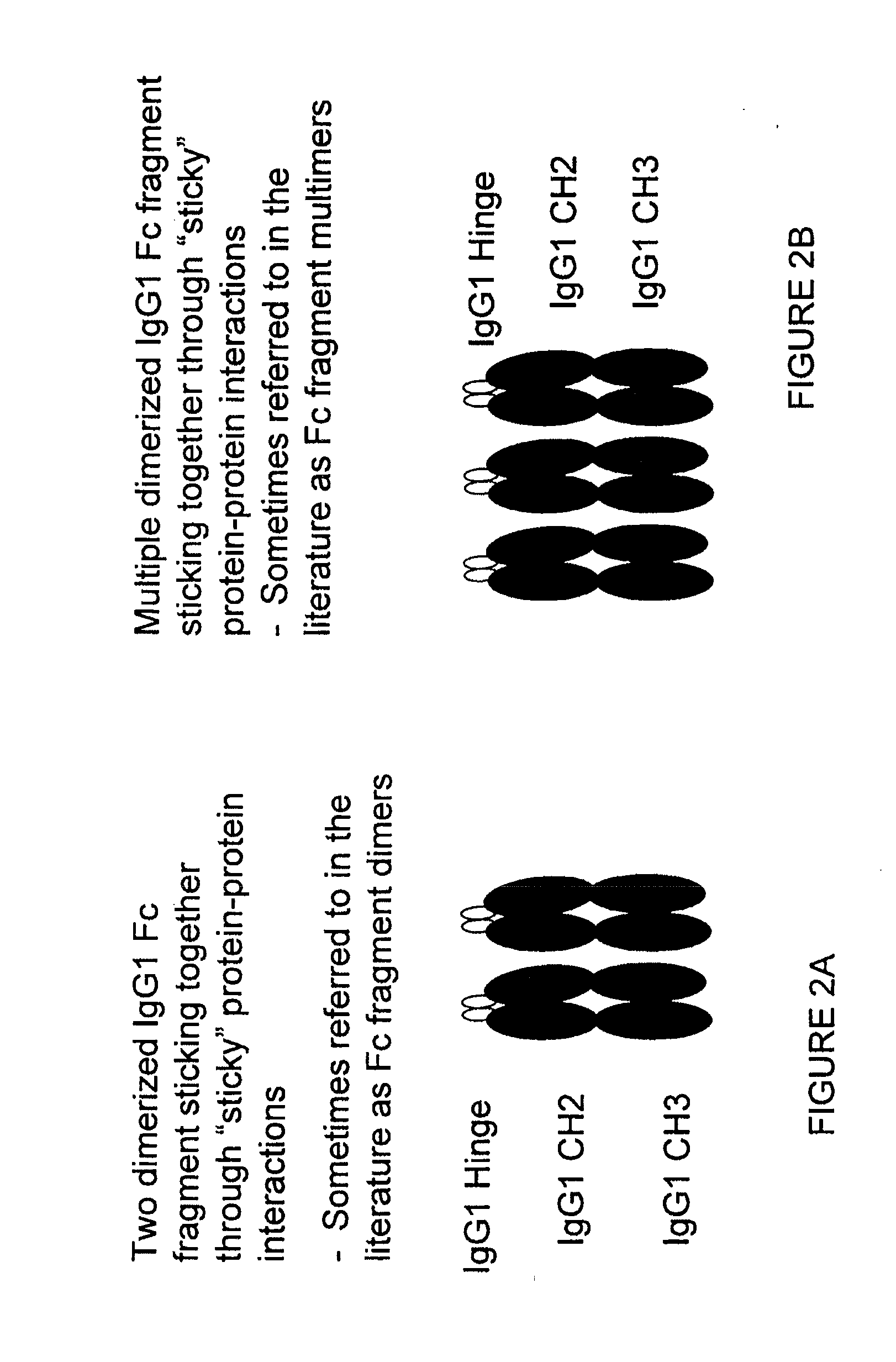
[0355]We have constructed stradomers of four different classes to mimic the effects of IVIG on immature DC. The serial stradomers, cluster stradomer units comprising cluster stradomers, core stradomer units comprising core stradomers, and Fc fragment stradomers shown below in Table 3 were each produced exce...
example 3
Heat Aggregated Biomimetics are More Potent than IVIG
[0380]A stradomer is a biologically active mimetic of aggregated immunoglobulin and especially of the aggregated Fc fragments of those immunoglobulin. In some instances, heat aggregation of the biomimetics described herein can increase biological activity. We conclude that heat-aggregated biomimetics as herein described can be as potent as IVIG.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com