Potential preparation method for highly-efficient recombinant HIV-1 CRF07-BC gp140 immunogen
A CRF07, HIV-1 technology, applied in the field of bioengineering, to achieve the effect of high antigen reactivity and good uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1. Construction of recombinant gp140 eukaryotic expression vector
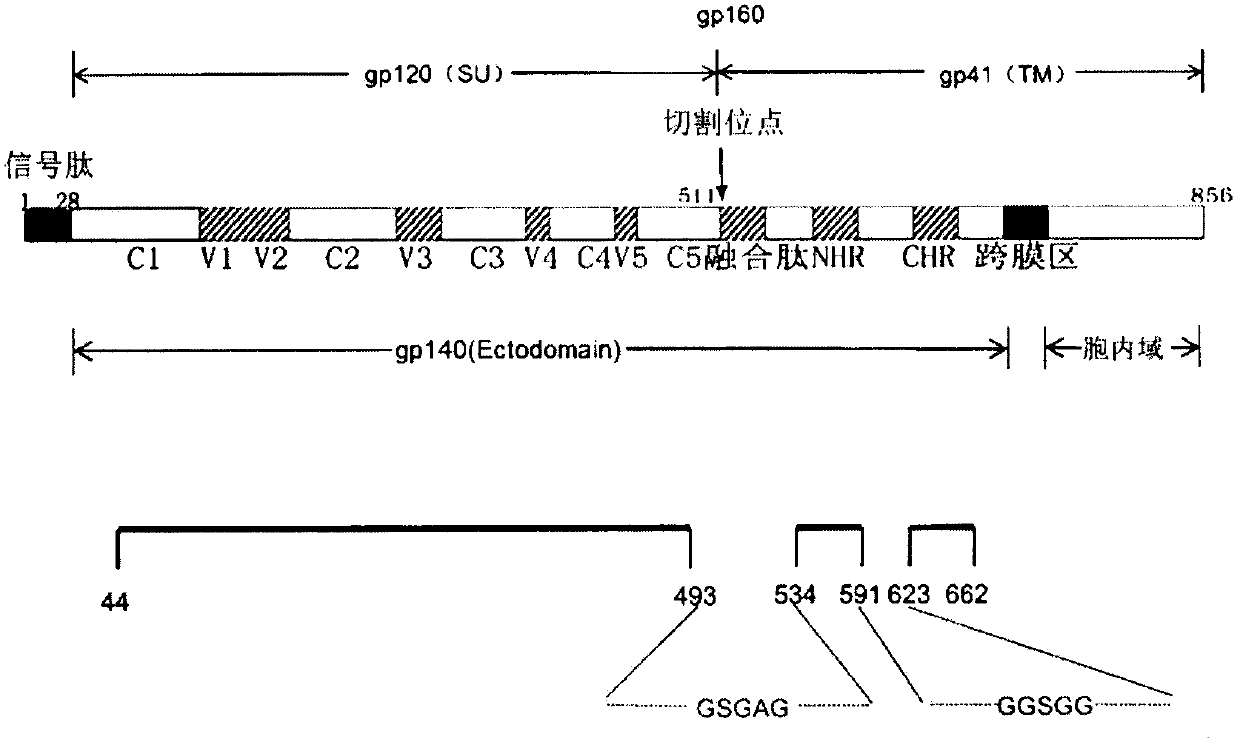
[0018] Using the CRF07-BC gp160 cDNA as a template, two overlapping extension PCRs were performed to obtain the extracellular domain of the envelope glycoprotein Env linked with two different linkers (see figure 1 ) DNA fragment gp140, the target gene fragment was cloned into the pMT / Bip / TEV-HisA vector modified by our laboratory by the method of double digestion and ligation. By replacing the V5 epitope with the cleavage site of tobacco etch virus protease (TEV enzyme), we can use TEV enzyme to remove the histidine tag (6×His-tag) at the carboxyl terminus of the target protein, so that the carboxyl group of the target protein can be removed. End with as few unnecessary oligopeptides as possible. After double-enzyme digestion and sequencing identification (see the sequence table for amino acid and nucleotide sequences), the target plasmid is extracted by an endotoxin-free plasmid extraction kit...
Embodiment 2
[0026] Example 2. Co-transfection of S2 cells and screening of S2 cell lines stably expressing target proteins
[0027] Cells were plated in T25 culture flasks one day in advance, and co-transfection was performed by liposome method when the confluency of cells reached 60-70%. The transfection step was carried out according to the instructions of the transfection reagent Cellfectin II, and it was noted that the mass ratio of the recombinant plasmid and the resistance screening plasmid pCoBlast was 19:1. After the cells were incubated at 27°C for 5 hours, the transfection solution was aspirated, 4 mL of fresh SFX-Insect medium was added, and the incubation was continued for 48 hours. The original medium was discarded, and SFX containing blasticidin (final concentration of 25ug / mL) was added. -Insect medium for resistance screening, unsuccessfully transfected cells will die a lot within a week after adding blasticidin for screening, and a small number of adherent cells will cont...
Embodiment 3
[0028] Example 3. Expression and purification of recombinant gp140
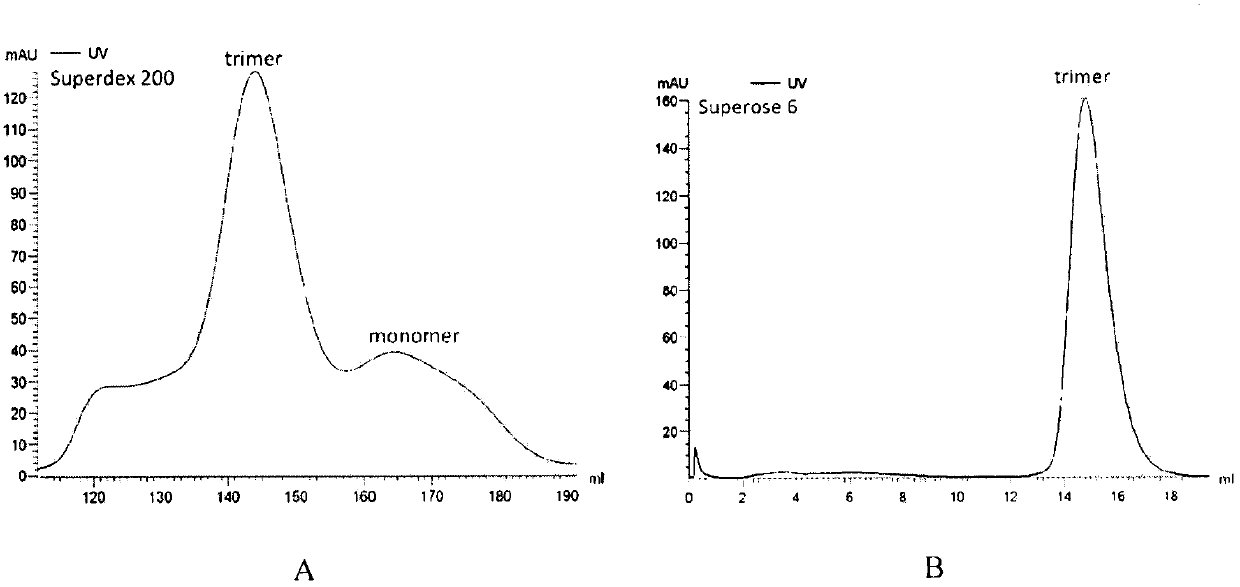
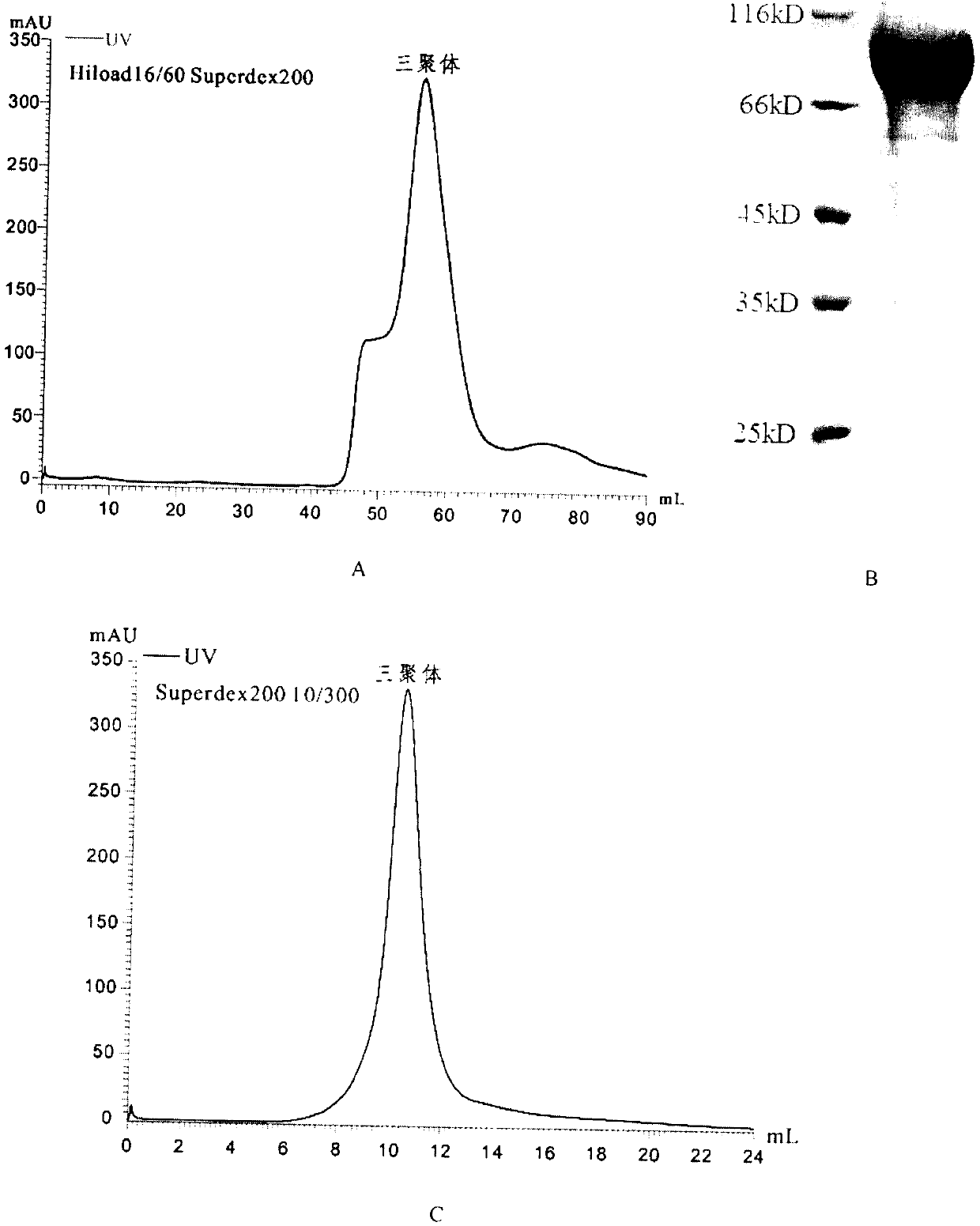
[0029] The selected T25S2 cell line was expanded into a T75 culture flask (volume ratio 1:5), and further transferred to a 500mL spinner flask for expanded culture (volume ratio 1:10), 27°C, 120rpm, and cultured for 2-3 days , when the growth of cells reached logarithmic phase, copper sulfate was added to induce protein expression (final concentration was 0.5 mmol / L), and the cells were collected after 3 days. The S2 cells were collected into a 300 mL centrifuge bucket, and centrifuged at 4000 rpm for 15 min at 4°C. The cell supernatant was collected, filtered through a 0.22 μm filter, and placed in an Amicon Stirred Cell8003 ultrafiltration cup to concentrate the protein. When compressed to 50 mL, the cells were combined with a nickel column binding buffer (50 mM / L Tris-HCl, 500 mM / L NaCl, After diluting 10 times with pH 8.0), the protein solution was collected into a 50 mL centrifuge tube, centrifuged at 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com