Envelope protein trimer immunogen capable of inducing HIV-1 broad spectrum and neutralizing antibody and application thereof
A technology of HIV-1 and envelope protein, applied in the field of biomedicine, can solve the problems of complex bnAb evolution mechanism and lack of mature experimental evidence, and achieve good immune results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
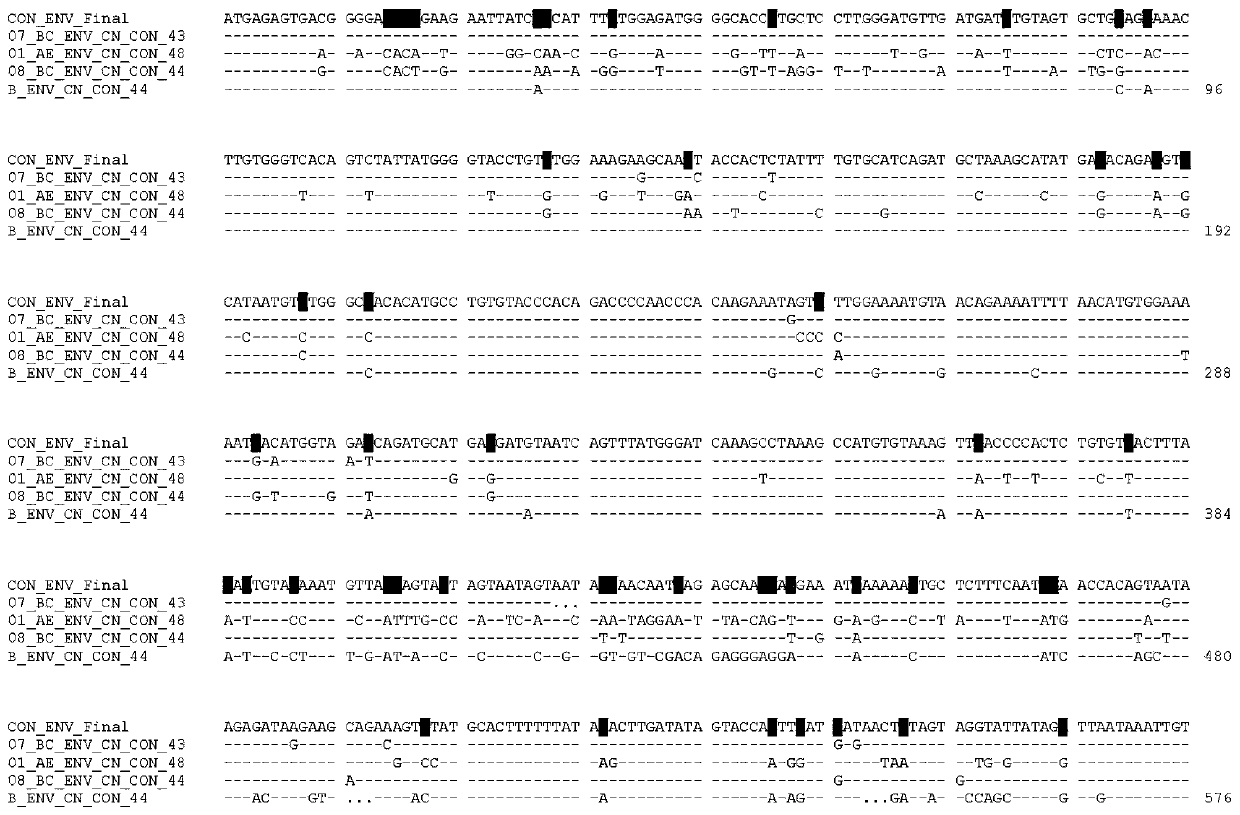
[0020] Example 1 Env consensus gene sequence design
[0021] 1. Obtain aligned sequences from the HIV sequence database (each sequence comes from an infected person)
[0022] 2. Delete the same sequence
[0023] 3. Delete sequences with more than 10 ambiguous bases
[0024] 4. Use Seaview software to manually adjust and prepare the optimal sequence alignment
[0025] 5. Determine the dominant nucleotide (>50%) for each position using the online tool with a 50% threshold. If some sites cannot determine the dominant nucleotide (50% or the highest dominant nucleotide <50%), then reduce the percentage of the threshold to obtain a clear dominant nucleotide (http: / / www.hiv.lanl .gov / content / sequence / CONSENSUS / AdvCon.html).
[0026] 6. Manually adjust according to the following rules to obtain the final consensus gene sequence:
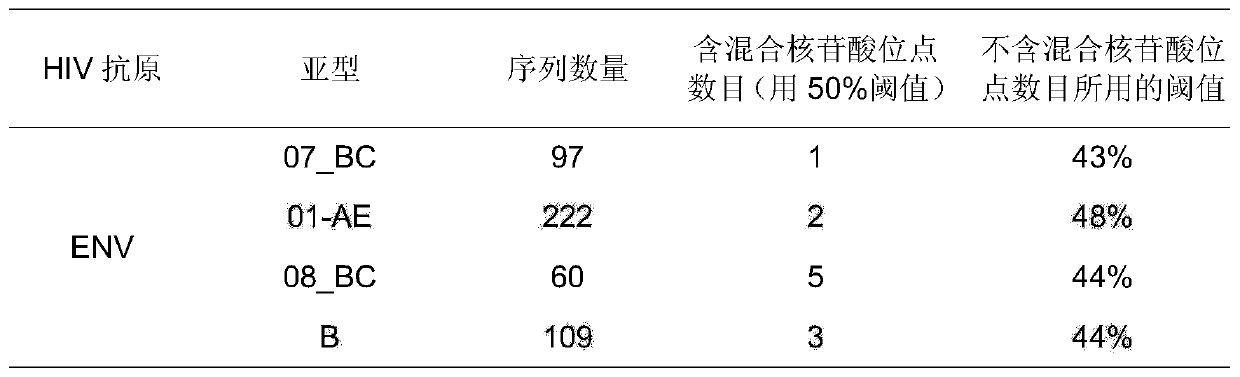
[0027] a. Main rule: Most nucleotides in the four CRF / subtypes were selected as the consensus gene sequence. CRF07_BC (35.5%), CRF01_AE (27.6%), CRF08...
Embodiment 2
[0029] Embodiment 2 Antigen protein expression vector construction
[0030] This program uses the pTT5 plasmid as the expression vector. Using the 718B.con Env gene sequence as a template, gp120con was amplified by PCR. At the same time, the fragments of gp120AE, tPA, Protan and MTQ were amplified using the plasmids stored in the laboratory as templates, and the homologous recombination kit was used to perform homologous recombination to obtain pTT5-tPA-Protan-gp120con-MTQ, pTT5-tPA-Protan- Antigen protein expression vectors such as gp120AE-MTQ and pTT5-tPA-gp120con-MTQ.
[0031] (1) Primer design:
[0032] tPA-F:CTGGCTAGCGTTTAAACTTAAGCTTGCCACCATGGATGCAATGAAGAGA
[0033] tPA-R: GCGGGTTTAAACGGGCCCCTTAGACTCGAGGGTACCGCTGGGCGAAACGAAGAC
[0034] Protan-F: CACTGGGCATCGCCCCAACTGGATCCGGTGGTGGT
[0035] Protan-R: GTTTAAACGGGCCCCTTAGACTCGAGTTATTTAATACGCA
[0036] gp120-F:AGTTTAAACGGATCTCTAGCGAATTCGCCACCATGGATGCAATGAAGAG
[0037] gp120-R:AGCCAGAGGTCGAGGTCGGGCTCGAGTTAAGTTGGGGCGATGC...
Embodiment 3
[0061] Example 3 Antigen protein expression and purification
[0062] The extracted plasmid was transiently transfected into 293-6E suspension cells, and the cell culture supernatant was harvested 72 hours later. BG505 UFO, CM, PAM and PCM trimeric proteins were obtained by affinity chromatography with lentil lectin and size exclusion chromatography with superdex 200, and then identified by protein electrophoresis. Specific steps are as follows:
[0063] (1) Transient transfection of 293-6E
[0064] 1. After recovery, the cells can be propagated to more than three passages. If the cells are in good condition, they can be transfected.
[0065] 2. Count the cells before transfection to ensure that the cell density is at 1.5xl0 6 cells / mL.
[0066] 3. Prepare the DNA / PEI complex, DNA:PEI=1:3, the final concentration of DNA is 1.25μg / 1×10 6 The cells were added to the new medium, and DNA and PEI (1 μg / μL) were added to 2 mL of the medium, and after incubation for 5 minutes, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com