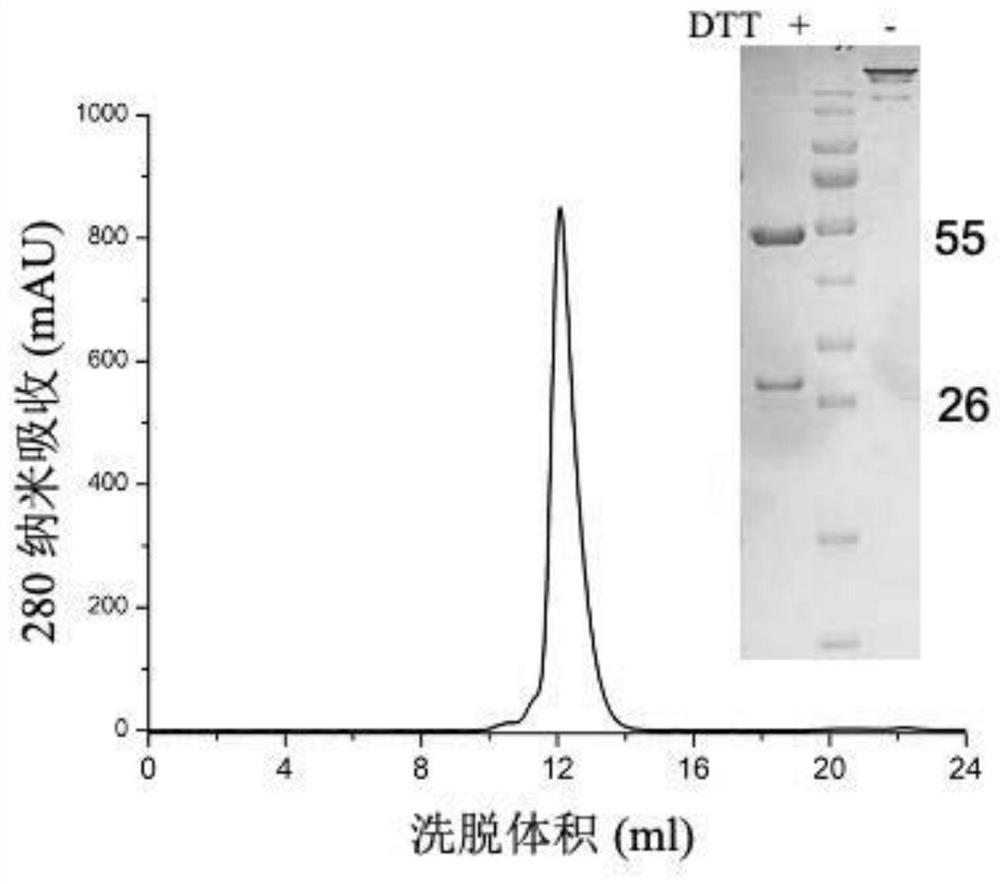
Human monoclonal antibody specifically binding to envelope protein Gn of severe fever with thrombocytopenia syndrome virus and application thereof
A technology for cloning antibodies and platelets, applied in the field of medicine, can solve problems such as no specific drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Expression and purification of the extracellular segment of SFTSV Gn
[0048]The coding sequence of 6 histidine tags (6×histag) and translation stop codons were connected to the 3' end of the coding region of the extracellular segment of the SFTSV Gn protein (SEQ ID NO: 9), and constructed into the pFastBac1 vector by EcoRI and XhoI (purchased from Invitrogen). The ligation product was then transformed into DH10Bac competent cells (purchased from Tiangen) for baculovirus recombination. The recombinant baculovirus was extracted, transfected into sf9 cells (purchased from Invitrogen) for baculovirus packaging, and then amplified by the virus, added to Hi5 cells (purchased from Invitrogen) for SFTSV Gn extracellular segment protein expression.
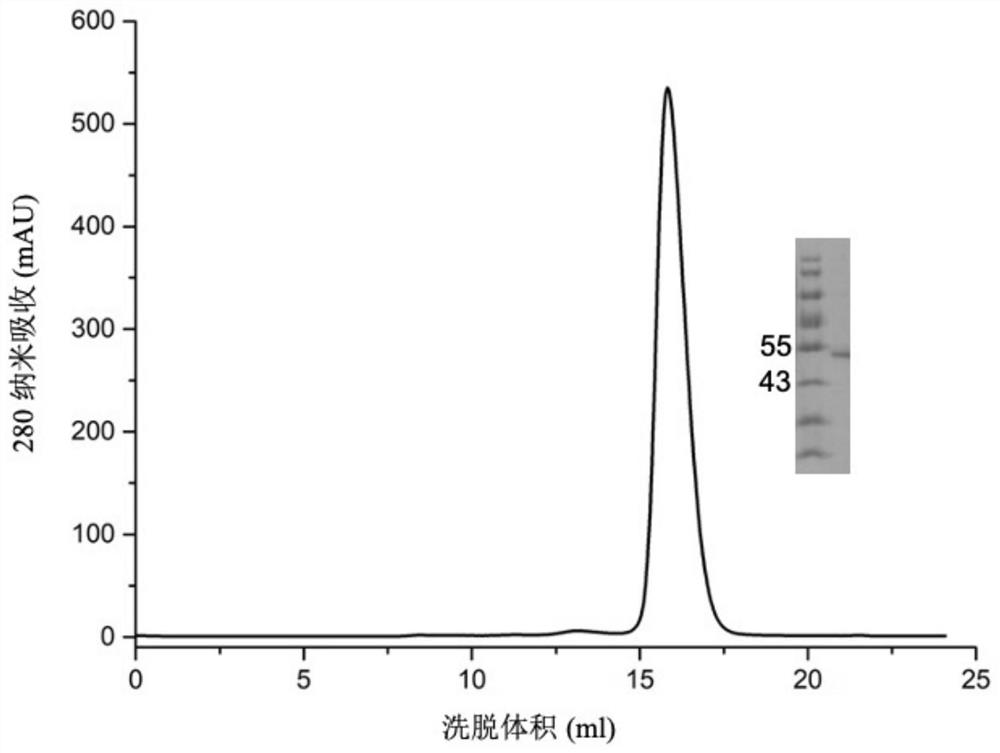
[0049] After the cell culture medium containing the target protein is purified by nickel ion affinity chromatography (HisTrapTMHP(GE)) and gel filtration chromatography (SuperoseTM6Increase 10 / 300GL(GE)), a relatively ...
Embodiment 2
[0050] Example 2: Isolation of SFTSV Gn protein-specific memory B cells
[0051] 15mL of blood was collected to isolate PBMCs with the informed consent of a person who was cured and discharged after SFTSV infection (1 person from Beijing Ditan Hospital Affiliated to Capital Medical University). The isolated PBMCs were divided into 10 7 The density of / mL was combined with the purified SFTSV Gn extracellular segment protein in Example 1 with a final concentration of 400nM, which was incubated on ice for half an hour, then washed twice with PBS, and then mixed with the following antibodies (all purchased from BD, using both 10μg / mL) were incubated with: anti-human CD3 / PE-Cy5, anti-human CD16 / PE-Cy5, anti-human CD235a / PE-Cy5, anti-human CD19 / APC-Cy7, anti-human CD27 / Pacific Blue, anti-human CD38 / APC, anti-human IgG / FITC, and anti-His / PE. After antibody incubation on ice for half an hour, PBMCs were washed twice with PBS.
[0052] PBS-washed PBMCs were sorted by FACSAria III to...
Embodiment 3
[0053] Example 3: Single B cell PCR, sequence analysis and human antibody design
[0054] According to the method described in Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus published by Qihui Wang et al. in Science Translational Medicine, Vol. 8, No. 369 in December 2016, the B cells obtained in Example 2 were treated with Reverse transcription was performed by Superscript III reverse transcriptase (Invitrogen), and the reverse transcription primers were as shown in Table 1, and the reaction was performed at 55°C for 60 minutes.
[0055] Table 1. Primers for reverse transcription reactions
[0056]
[0057] Using this reverse transcription product as a template, PCR was performed with HotStar Tap Plus enzyme (QIAgen) to amplify the antibody variable region sequence (PCRa). The corresponding primers were designed, and the reaction conditions were as follows: 95°C, 5 min; 95°C for 30s, 55°C (heavy chain / κ chain) for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com