Quantitative determination kit for neutralizing antibodies of virus and application thereof
A quantitative detection and kit technology, applied in the biological field, can solve the problems of increasing the difficulty of neutralizing antibodies, inconspicuous cell lesions, laborious and other problems, and achieve the effect of shortening the detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of NP protein monoclonal antibody and polyclonal antibody
[0025] Amplify the SFTS virus NP gene by RT-PCR, insert the reading frame into the multiple cloning site of the expression plasmid pQE30 (Qiagen, Germany), obtain the prokaryotic expression plasmid pQE30-SFTS-NP of the NP gene, and transform E.Coli M15 bacterial strain (Qiagen , Germany), induced expression of 6His-NP recombinant protein. After the NP recombinant protein was purified and quantified, BALB / c mice were immunized according to conventional methods to prepare monoclonal and polyclonal antibodies against SFTS virus NP protein.
Embodiment 2
[0026] Embodiment 2:. Determination of virus titer
[0027] Tissue cell half infectious dose (TCID50) assay was used. The virus solution frozen at -70°C was diluted 100 times. Take the 1:100-fold diluted virus liquid, and perform 1 / 2 log dilution on a 96-well cell culture plate, that is, 10 -2 、10 -2.5 、10 -3 、10 -3.5 ...10 -7 . Each well contains 100 μl of virus solution, and each dilution is repeated in 4 wells.
[0028] Take the cells in the logarithmic growth phase, digest with LEDTA-trypsin, disperse the cells with cell culture medium, add virus dilution to 10mL, count the cells with a cell counting plate, and dilute the cells to 1.5×10 5 cells / mL for later use. Add 100 μl cells (1.5x10 4 cells / well). It is best to use one column of the cell culture plate as the normal cell (CC) control. Cultivate at 37°C, 5% CO2 incubator for 20-24h.
[0029] The cells were washed twice with PBS (pH 7.2), 100 μl of 80% ice acetone was added to each well, and the cells were fi...
Embodiment 3
[0031] Example 3: Detection of neutralizing antibodies
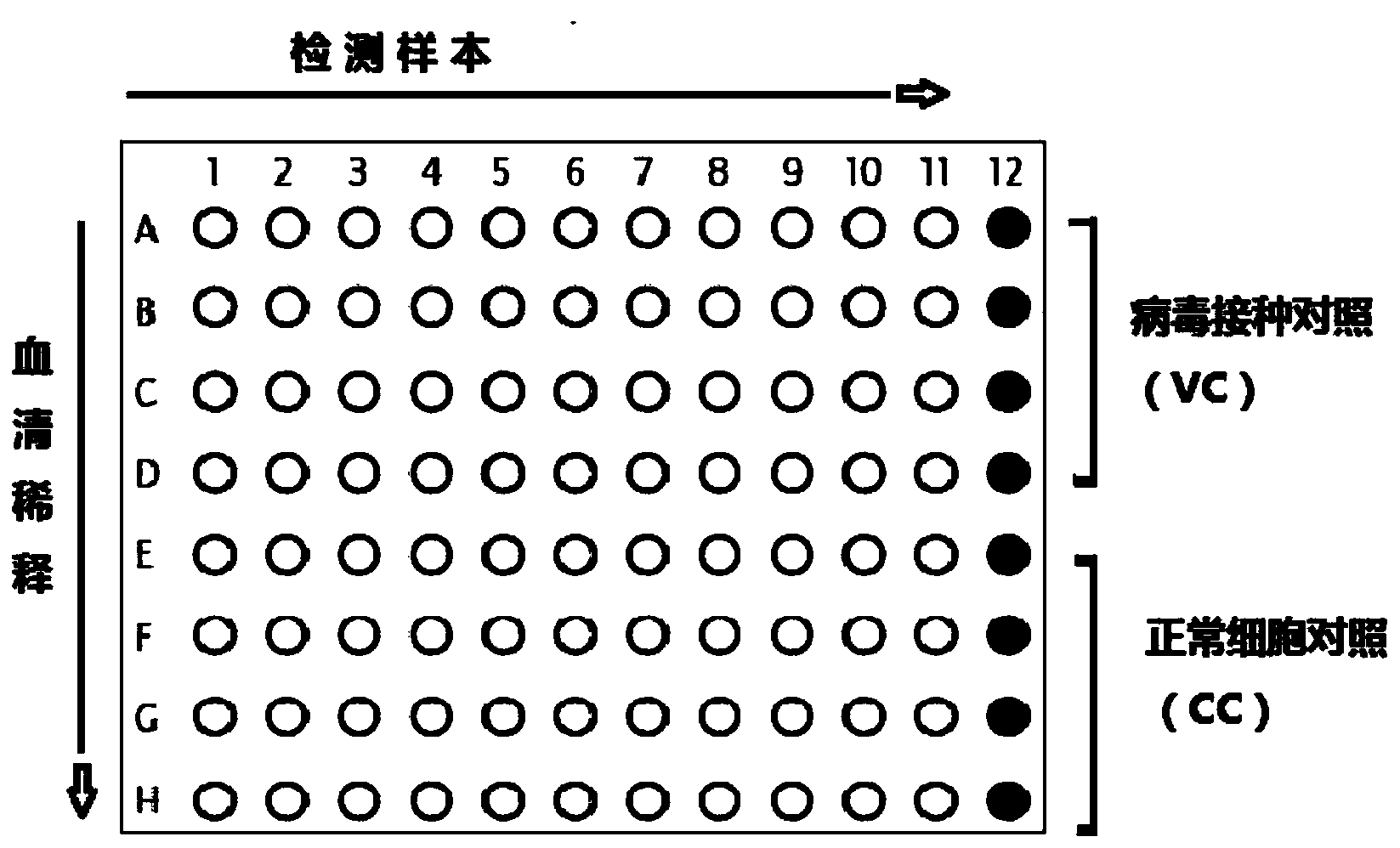
[0032] Serum neutralizing antibody titers were tested on 96-well cell culture plates. Add 50 μl of MEM cell culture medium (without bovine serum) to each well of a 96-well cell plate. In the first 11 wells (A1~A11) in the first row, add 40 μl of virus diluent to make it 90 μl / well, add 10 μl of the serum to be tested in the first row A1~A11, and the serial dilution (A~H) is 1:10, 1:20, 1:40...1:1280. Dilute virus to 100TCID 50 / 50mL. Add 50 μl virus working solution to each well except CC wells (E12, F12, G12, H12). Wells A12, B12, C12 and D12 were used as virus inoculated control (VC) wells. Add 50 μl of MEM cell culture medium (without bovine serum) to CC wells. In addition, select 1 column of wells to verify the titer of the virus working solution, add 200TCID50 / 100mL to each well, and make serial doubling dilutions to make it 100TCID50, 50, 25, 12, 6,...0.7, and then add 50μl to each well The volume of the MEM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com