ELISA (enzyme-linked immunosorbent assay) kit for EV (enterovirus) 71 inactivated vaccine antigen
An enzyme-linked immunosorbent assay and enterovirus technology, which is applied in the biological field, can solve problems such as the inability to correctly detect the antigen content of inactivated vaccines, the influence of test results, and the inability to monitor vaccines, and achieves good specificity, high sensitivity, and good specificity. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
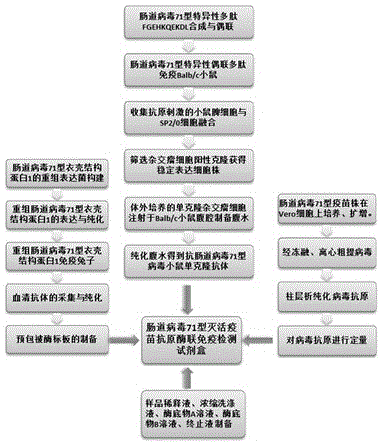
[0028] Example 1 Preparation of anti-enterovirus 71 virus polyclonal antibody
[0029] 1) The enterovirus type 71 capsid structural protein (VP1) expression gene was cloned to obtain a recombinant expression bacterium expressing the enterovirus type 71 capsid structural protein;
[0030] 2) Express enterovirus type 71 capsid structural protein (VP1) recombinant antigen with the obtained recombinant bacteria expression bacteria, and obtain relatively pure recombinant antigen through column chromatography;
[0031] 3) Rabbits were immunized with the obtained antigen, immunized four times at intervals of one week, each immunization was 0.5 mg. Serum was collected four weeks after the last immunization;
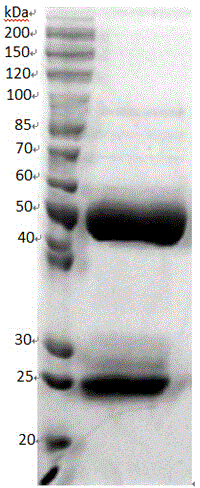
[0032] 4) The obtained serum was purified by protein A column chromatography to obtain the purified anti-enterovirus 71 polyclonal antibody. The resulting purified antibody see figure 2 .
Embodiment 2
[0033] Example 2 Preparation of anti-enterovirus 71 virus mouse monoclonal antibody
[0034] 1) Enterovirus 71-specific polypeptide FGEHKQEKDL synthesized, and coupled with keyhole limpet hemocyanin carrier protein;
[0035] 2) The resulting conjugated protein was immunized with Balb / c mice to obtain antigen-stimulated mouse splenocytes;
[0036] 3) The splenocytes were fused with mouse myeloma cells (SP2 / 0), and screened by indirect ELISA
[0037] Positive clones of hybridoma cells secreting specific antibodies, and then subcloned and identified by limiting dilution
[0038] IgG subtype, obtain a stable monoclonal hybridoma cell line;
[0039] 4) Ascites was prepared by injecting monoclonal hybridoma cells cultured in vitro into the peritoneal cavity of Balb / c mice, and the obtained ascites was chromatographed on a protein A column to separate and obtain purified anti-enterovirus 71 monoclonal antibodies. The resulting purified antibody see image 3 .
Embodiment 3
[0040] Example 3 Preparation of enterovirus 71 virus antigen standard
[0041] 1) The enterovirus 71 antigen standard product is obtained by culturing and amplifying the enterovirus 71 vaccine strain on Vero cells, freeze-thawing, centrifugal extraction and column chromatography. It is used for the calibration of the antigen content of enterovirus 71 inactivated vaccine. See the resulting antigen Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com