The testing method of adenosine deaminase
A technology for detecting reagents and purine nucleoside phosphorylase, which is applied in the field of medical testing, can solve problems such as large differences in test results, unfavorable clinical test results, and troubles in clinical diagnosis and treatment, and achieve stable detection methods, overcome non-specific reactions, and clinically The effect of medical convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1. Reagent composition and concentration
[0067] Reagent 1: Tris-HCl (80.0mmol / L, pH6.6), disodium hydrogen phosphate (Na 2 HPO 4 , 10.3mmol / L), 4-aminoantipyrine (4-AA, 1.8mmol / L), purine nucleoside phosphorylase (PNP, 37°C 2760U / L), xanthine oxidase (XOD, 37°C 600U / L), peroxidase (POD, 590U / L at 37°C), ascorbate (2306U / L at 37°C), NaN 3 (0.2mmol / L), TritonX-100 (0.76% of the total volume of reagent 1), EDTA (0.2mmol / L);
[0068] Reagent 2 (starting reagent): Tris-HCl (80.0 mmol / L, pH 6.6), adenosine (12.2 mmol / L), EHSPT (2.0 mmol / L).
[0069] 2. Detection conditions
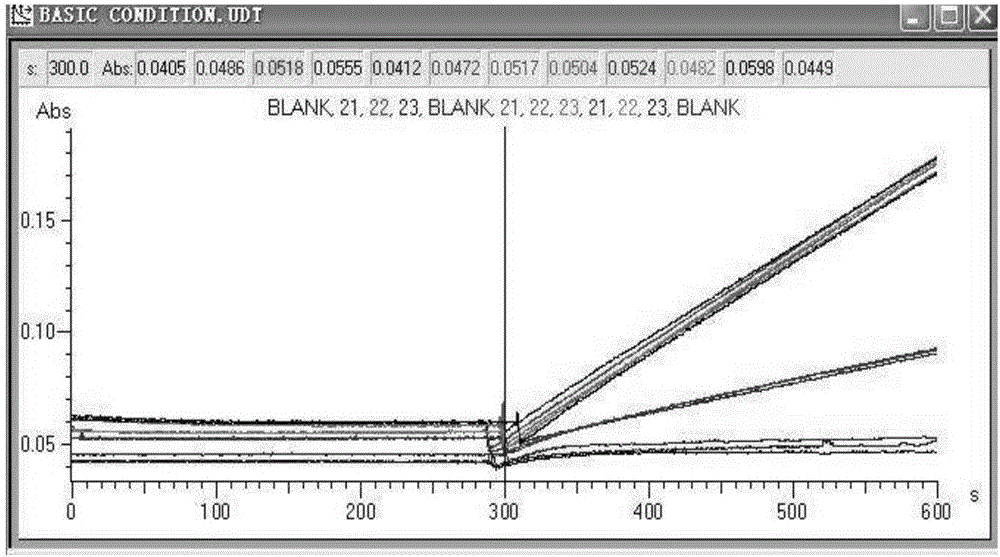
[0070]Detection temperature: 37±0.1°C; detection wavelength: 550±1nm; wave width: 10mm±0.01mm, optical path ≤ 2nm, detection points ≥ 6; incubation time: 300s; delay time: 120s; monitoring time: 180s.
[0071] 3. Test sample: serum.
[0072] 4. A detection method for adenosine deaminase, comprising the following steps:
[0073] 1) Add 180ul reagent 1 and 5ul serum samples into the cuvette, mix we...
Embodiment 2
[0079] The performance parameter that embodiment 2 adenosine deaminase detects
[0080] The performance parameters of the present technology were determined according to the method of Example 1.
[0081] The result is as follows:
[0082] Intra-assay precision: ≤1%, period precision: ≤2%;
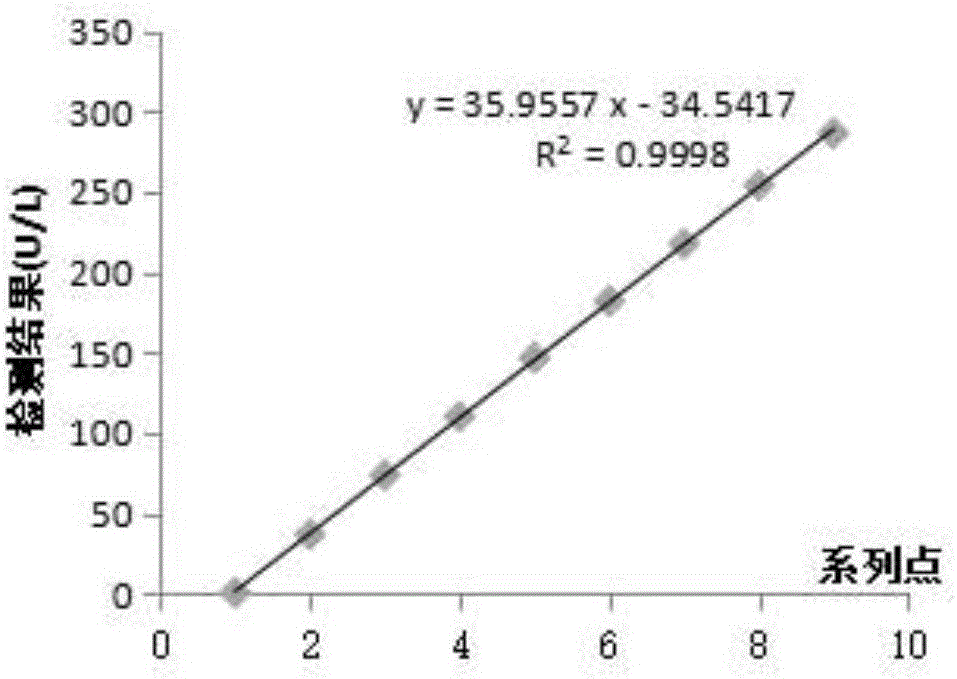
[0083] Linear range: 0.3-286U / L;
[0084] Sensitivity: The activity of the detection standard substance exceeds the given target value by about 30%.
[0085] Anti-interference ability: triglyceride ≤ 3.39mmol / L (about 20 times the normal adult level), hemoglobin ≤ 3.75g / L (visible hemolysis is 300mg / dl), bilirubin < 85.5umol / L, vitamin C ≤ 40mg / L (the content of vitamin C in normal human serum is ng / dl level), glycyrrhetinic acid and deoxycholic acid ≤ 20mg / L, baicalein ≤ 37.5mg / L will not interfere with the method.
[0086] The linear range diagram of the detection method is shown in the appendix figure 2 . figure 2 The linear coefficient of the curve is significant, and the correl...
Embodiment 3
[0088] Embodiment 3 repeatability test
[0089] Next, the method established by the present invention is tested for repeatability, and the intra-batch precision and period precision are calculated respectively.
[0090] Serum samples (high and low concentrations) with no visible lipemia, hemolysis, jaundice and negative infectious indicators were selected. Intra-batch precision: each sample is tested 10 times in a batch, and the mean, standard deviation and coefficient of variation (CV) are calculated. The precision during the period is: each sample is tested 3 times a day, and the mean and standard deviation are calculated for 12 consecutive days. and coefficient of variation (CV).
[0091] The results were intra-assay precision: ≤ 1%, inter-assay precision: ≤ 2%.
[0092] The intra-assay and inter-interval repeatability of the method are good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com