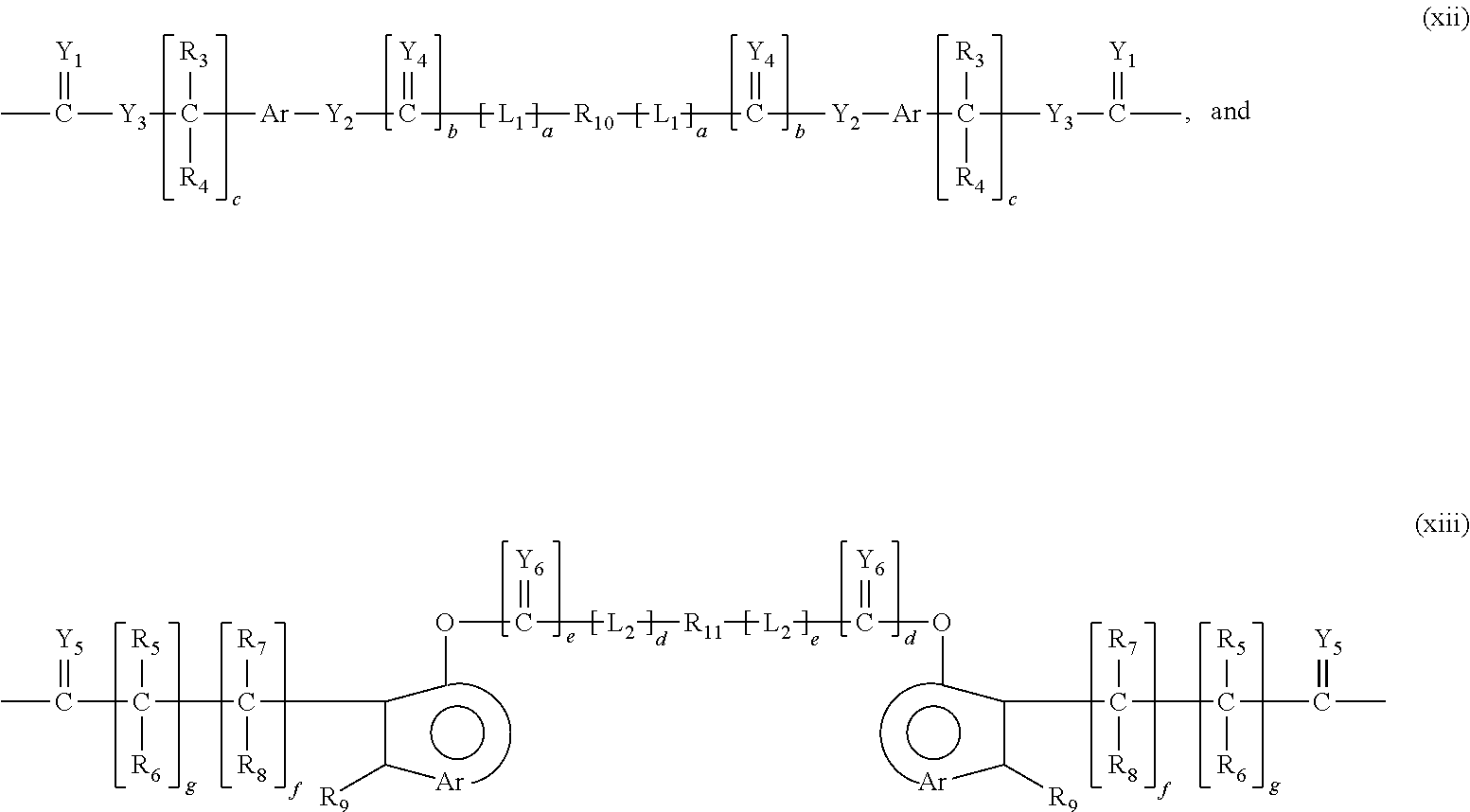
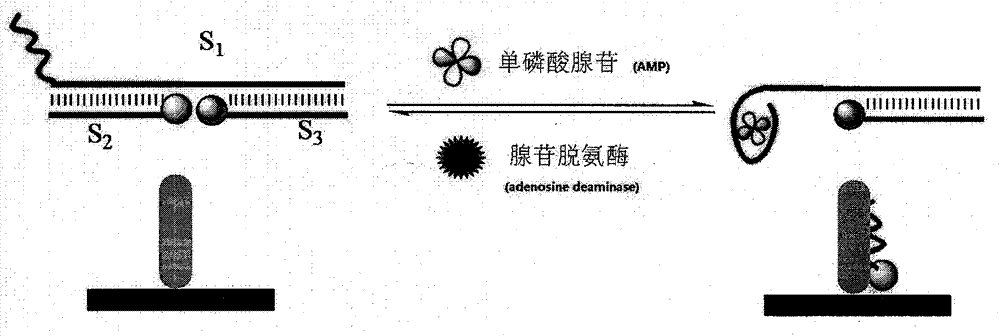
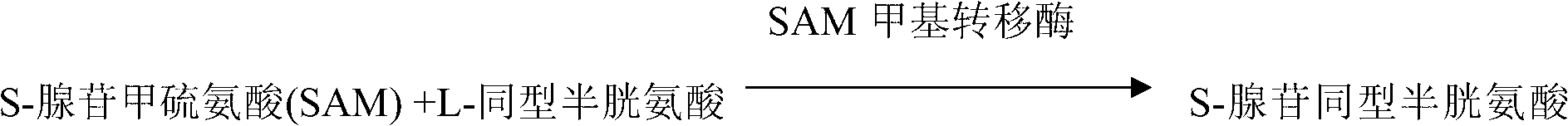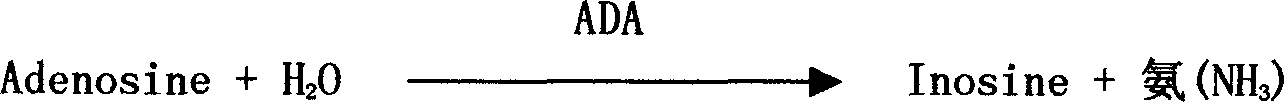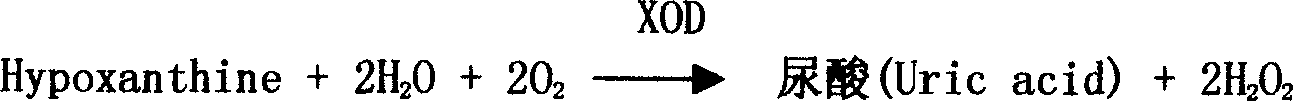Patents
Literature
149 results about "Adenosine deaminase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
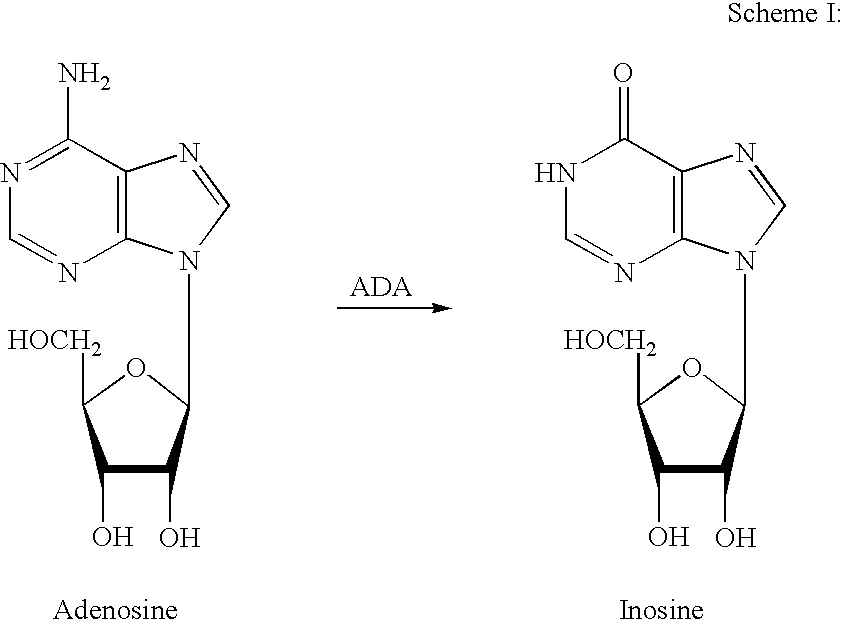
Adenosine deaminase (also known as adenosine aminohydrolase, or ADA) is an enzyme (EC 3.5.4.4) involved in purine metabolism. It is needed for the breakdown of adenosine from food and for the turnover of nucleic acids in tissues.
Adenosine deaminase deficient transgenic mice and methods for the use thereof
InactiveUS6207876B1Determining effectGuaranteed flatnessVectorsTissue cultureMedicineDeficient mouse
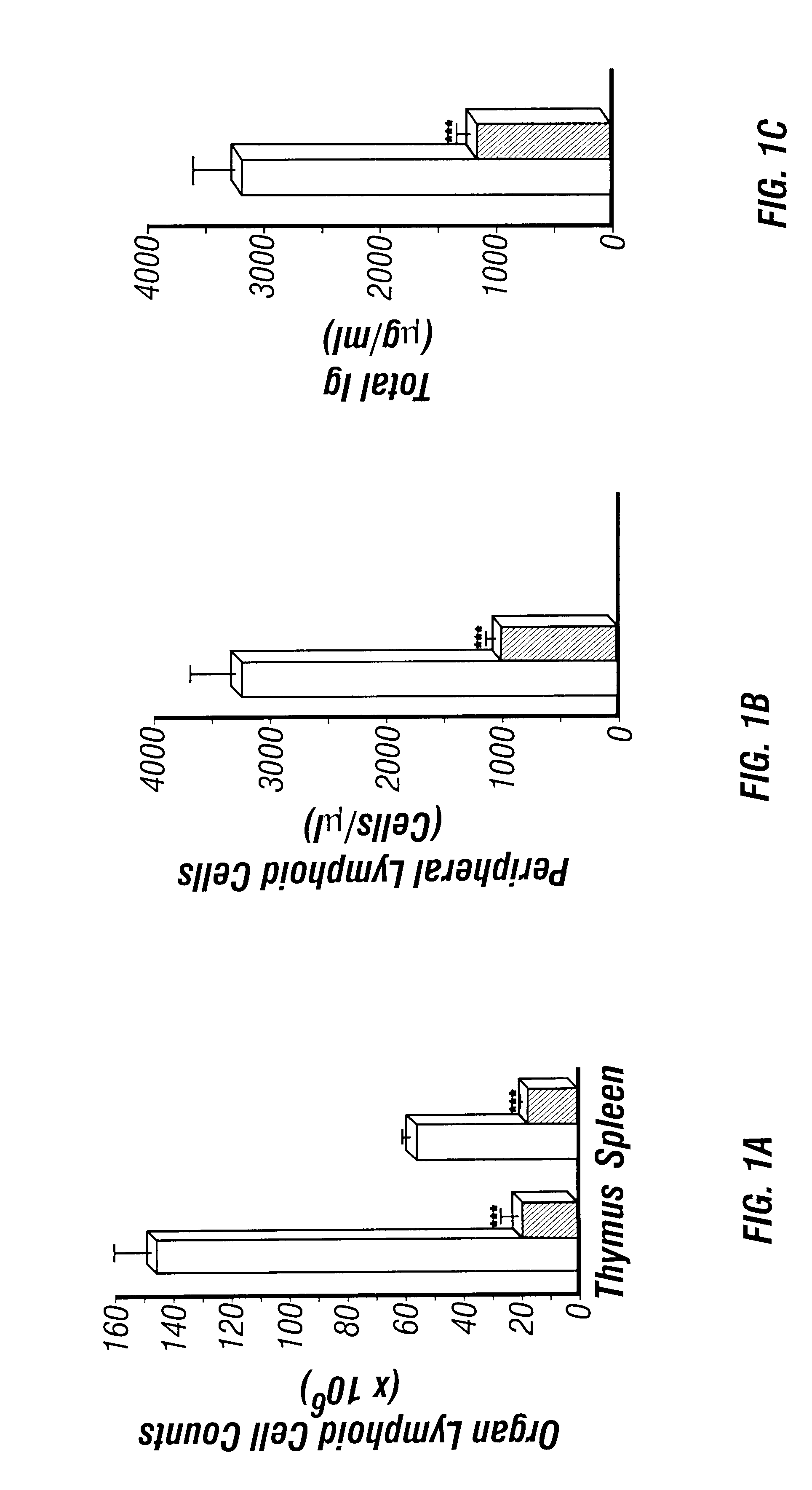
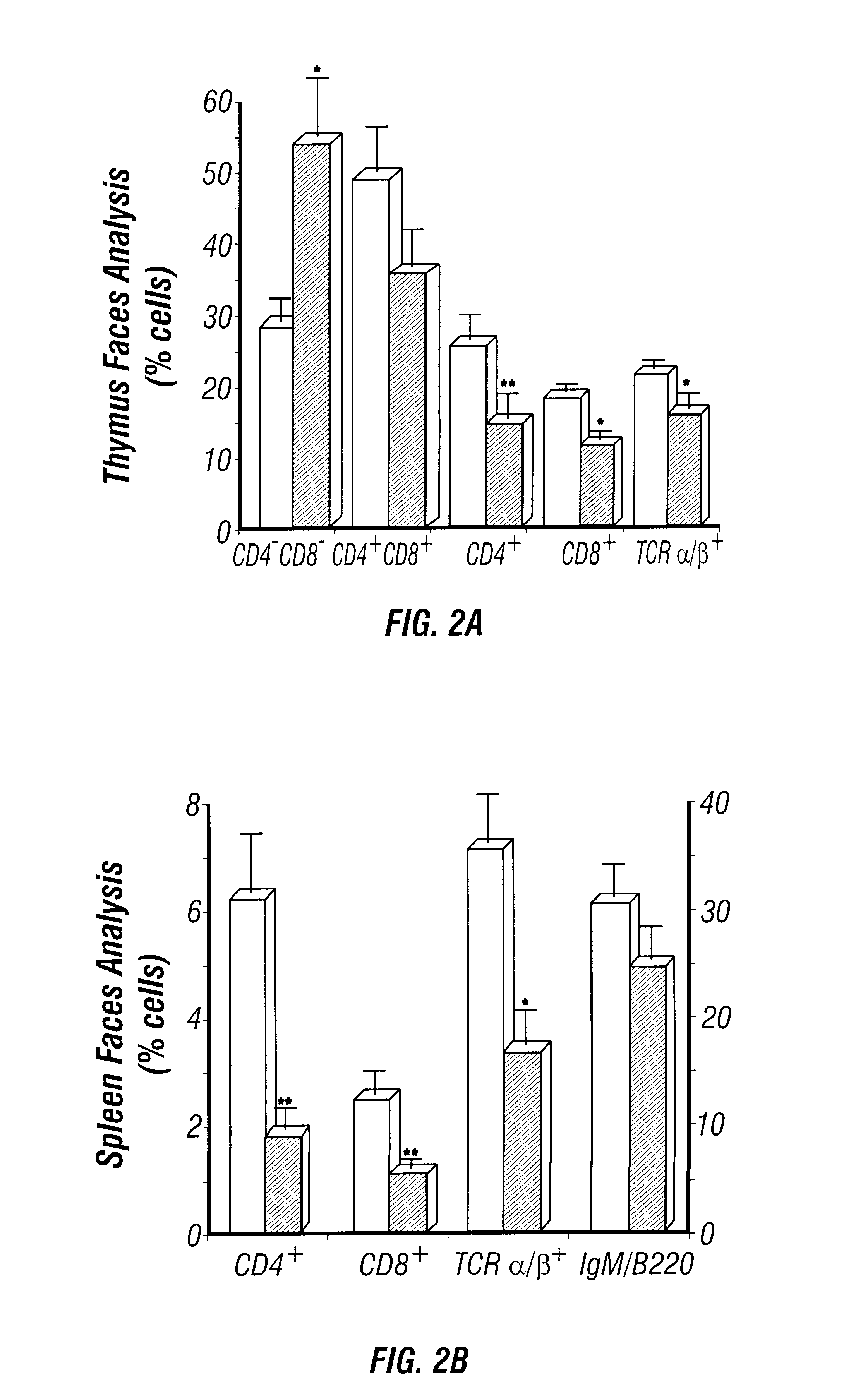
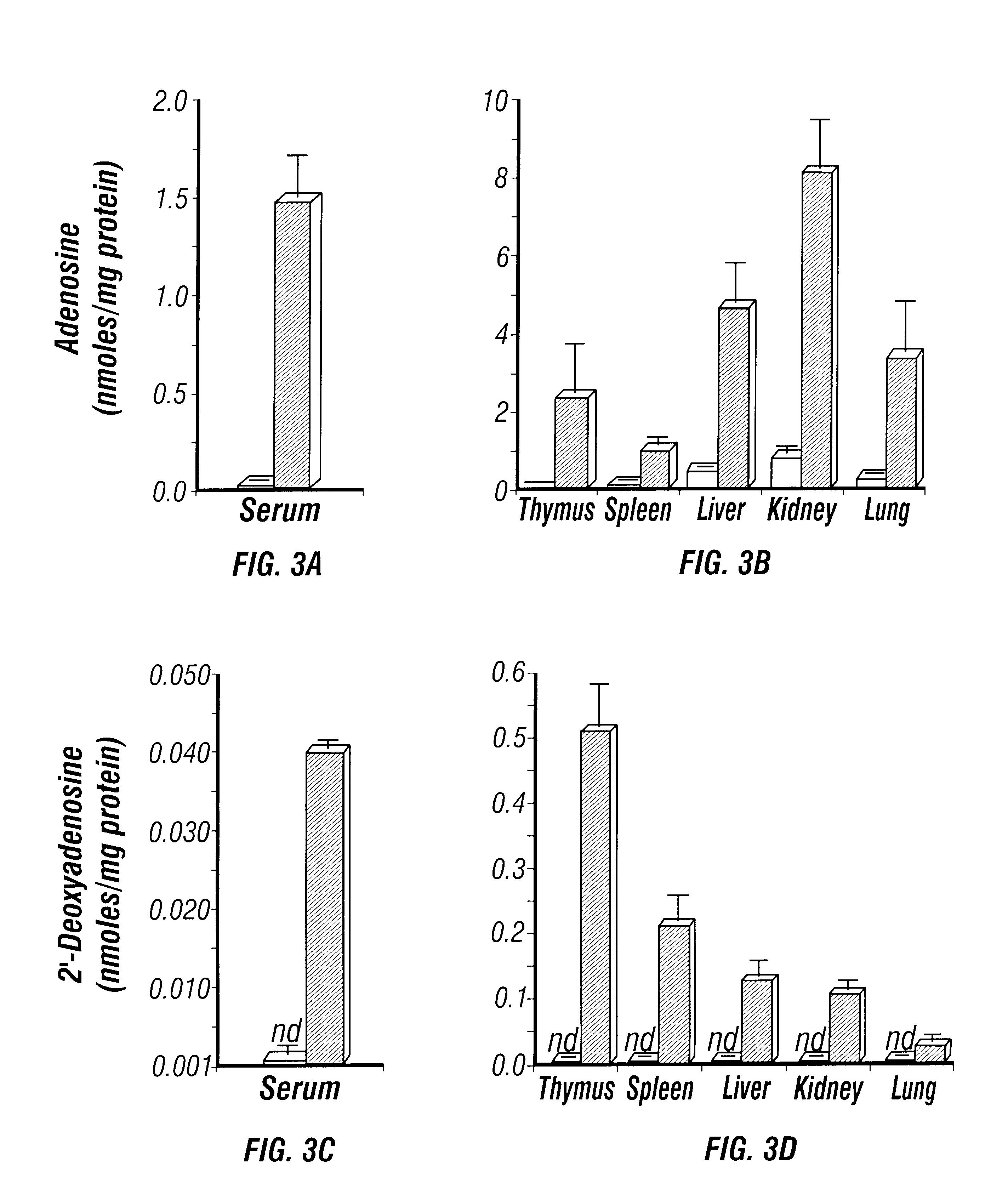
The present invention relates to the production of adenosine deaminase (ADA) deficient mice and the use of such mice as an animal model for dysfunctions associated with elevated adenosine levels. Also, provided by the present invention are methods of treating dysfunctions associated with elevated adenosine levels and methods of screening compounds for pharmaceutical activity in the treatment of dysfunctions associated with elevated adenosine levels.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Enzymatic fluorimetric assay of camp and adenylate cyclase
InactiveUS6762026B1Easy to operateReaction period can be extremely shortenedMicrobiological testing/measurementMaterial analysisRadioactive agentFluorescence
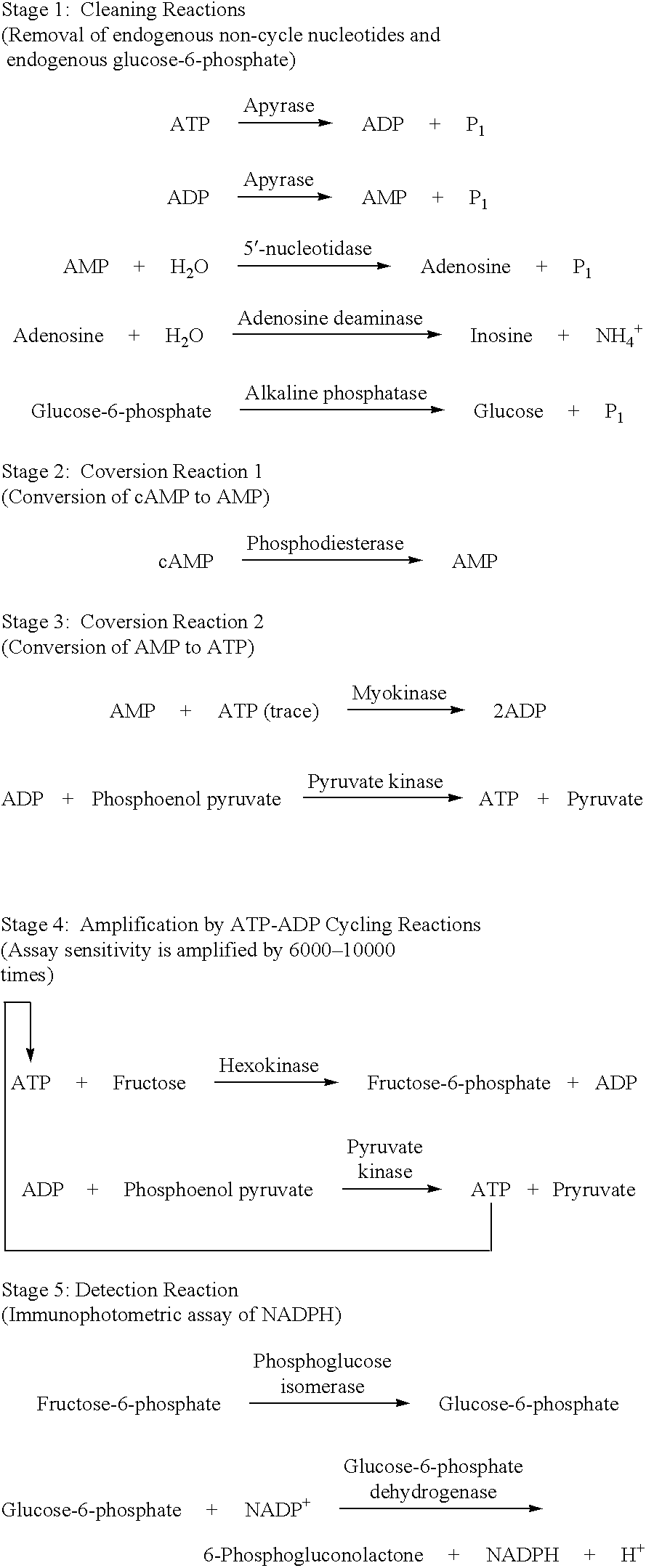
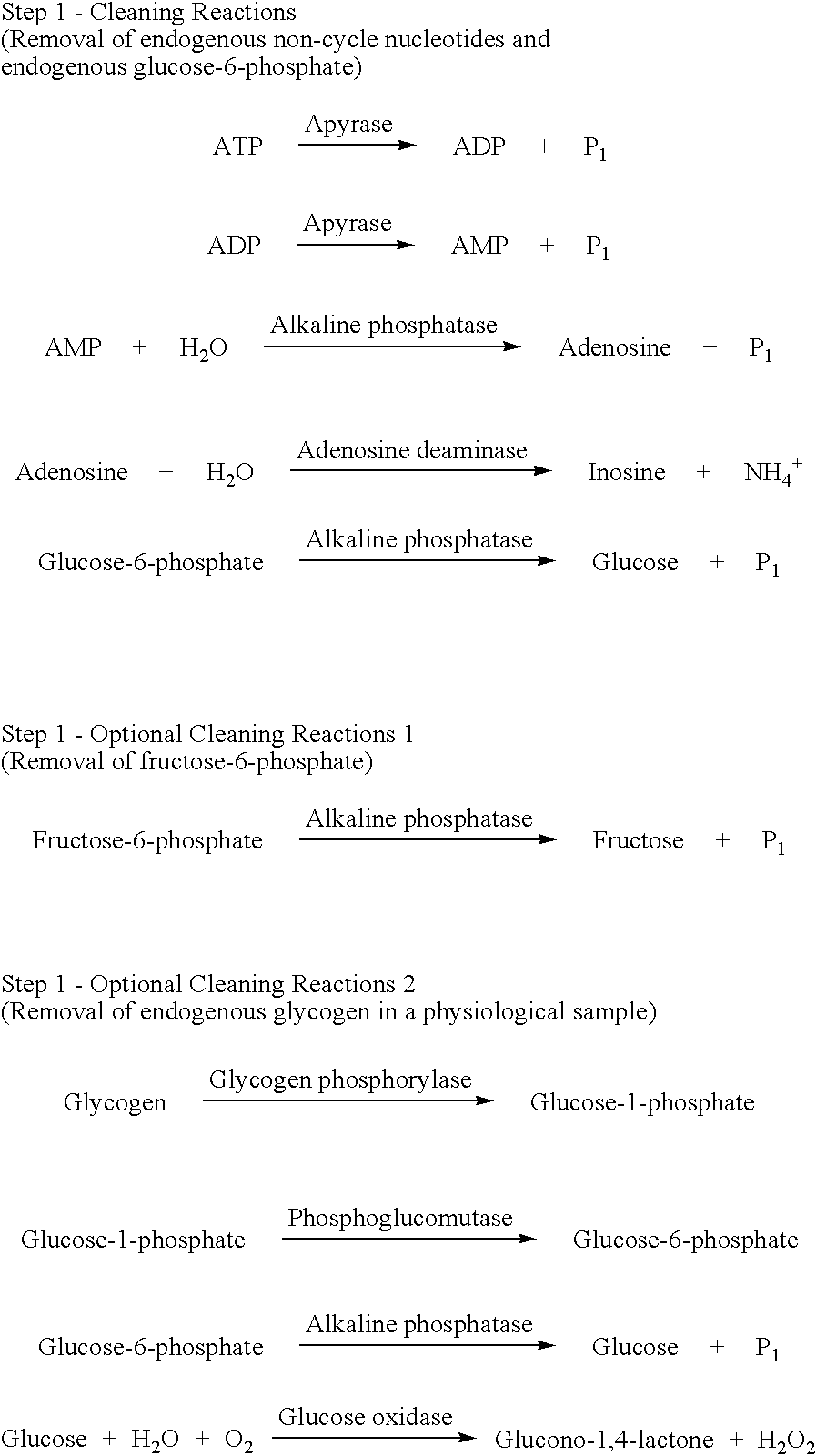
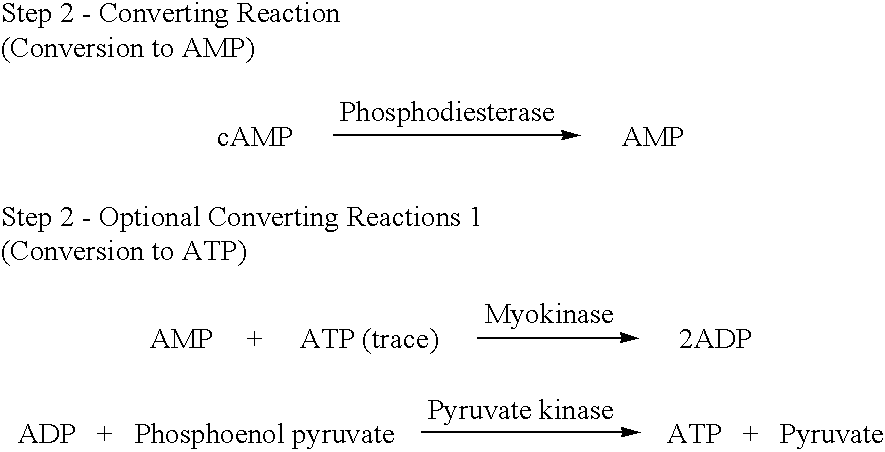
The present invention relates to a method for quickly determining cAMP content or an adenylate cyclase activity in a biological sample containing non-cyclic adenine nucleotides without the use of radioactive agents.Particularly, the present invention provides a method of determining cAMP content or an adenylate cyclase activity in a biological sample containing non-cyclic adenine nucleotides selected from the group consisting of cAMP produced by endogenous adenylate cyclase, and AMP, ATP, ADP and a mixture thereof, which comprises (1) combining a biological sample with effective amounts of apyrase, adenosine deaminase and alkaline phosphatase to enzymatically remove non-cyclic adenine nucleotides other than cAMP, and glucose-6-phosphate in the sample; (2) enzymatically converting cAMP into AMP; (3) determining an amount of AMP without the use of radioactive agents, and a kit to carry out the method.
Owner:FUSO PHARMA INDS
Adenosine deaminase deficient transgenic mice and methods for the use thereof
The present invention relates to the production of adenosine deaminase (ADA) deficient mice and the use of such mice as an animal model for dysfunctions associated with elevated adenosine levels. Also, provided by the present invention are methods of treating dysfunctions associated with elevated adenosine levels and methods of screening compounds for pharmaceutical activity in the treatment of dysfunctions associated with elevated adenosine levels.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
RNA site-directed editing technology based on CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas13a
The invention relates to a site-directed RNA (Ribonucleic Acid) editing technology based on CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas13a, and discloses an RNA site-directed editing method based on the CRISPR-Cas13a. By use of the method disclosed by the invention, during RNA site-directed editing, under the guidance of a crRNA, a Cas13a mutant takes a catalytic structural domain of ADAR2 (Adenosine Deaminase Acting on RNA 2) to an appointed RNA position to finish the site-directed editing from A to I. The Cas13a mutant does not have ssRNA cleavage activity, has ssRNA binding activity and is blended with the catalytic structural domain of the ADAR2. On the basis, the design of the crRNA is also optimized.
Owner:CAS CENT FOR EXCELLENCE IN MOLECULAR PLANT SCI
Adenosine A3 receptor agonist
InactiveUS20030143282A1Sugar derivativesPeptide/protein ingredientsLiquid mediumAdenosine A3 Receptor Agonists
The present invention relates to a naturally occurring low molecular weight adenosine A3 receptor agonist (LMW-A3RAg) which is preferably obtained from a vertebrate tissue or a vertebrate-derived cell by extraction in a liquid medium. The LMW-A3RAg of the invention is characterized by the following feature: (i) it is obtainable from animal-derived tissue or cells; (ii) it filters through a filter with a maximal molecular weight cut-off of about 3,000 Daltons; (iii) it is water soluble, heat stable, non-proteinaceous and resistant to adenosine deaminase activity. The invention also concerns pharmaceutical compositions comprising the naturally occurring LMW-A3RAg of the invention and therapeutic methods comprising administering to a subject in need an effective amount of the naturally occurring A3RAg for achieving a therapeutic effect, the therapeutic effect comprises inhibition of adenylate cyclase in target cells.
Owner:CAN-FITE BIOPHARMA LTD
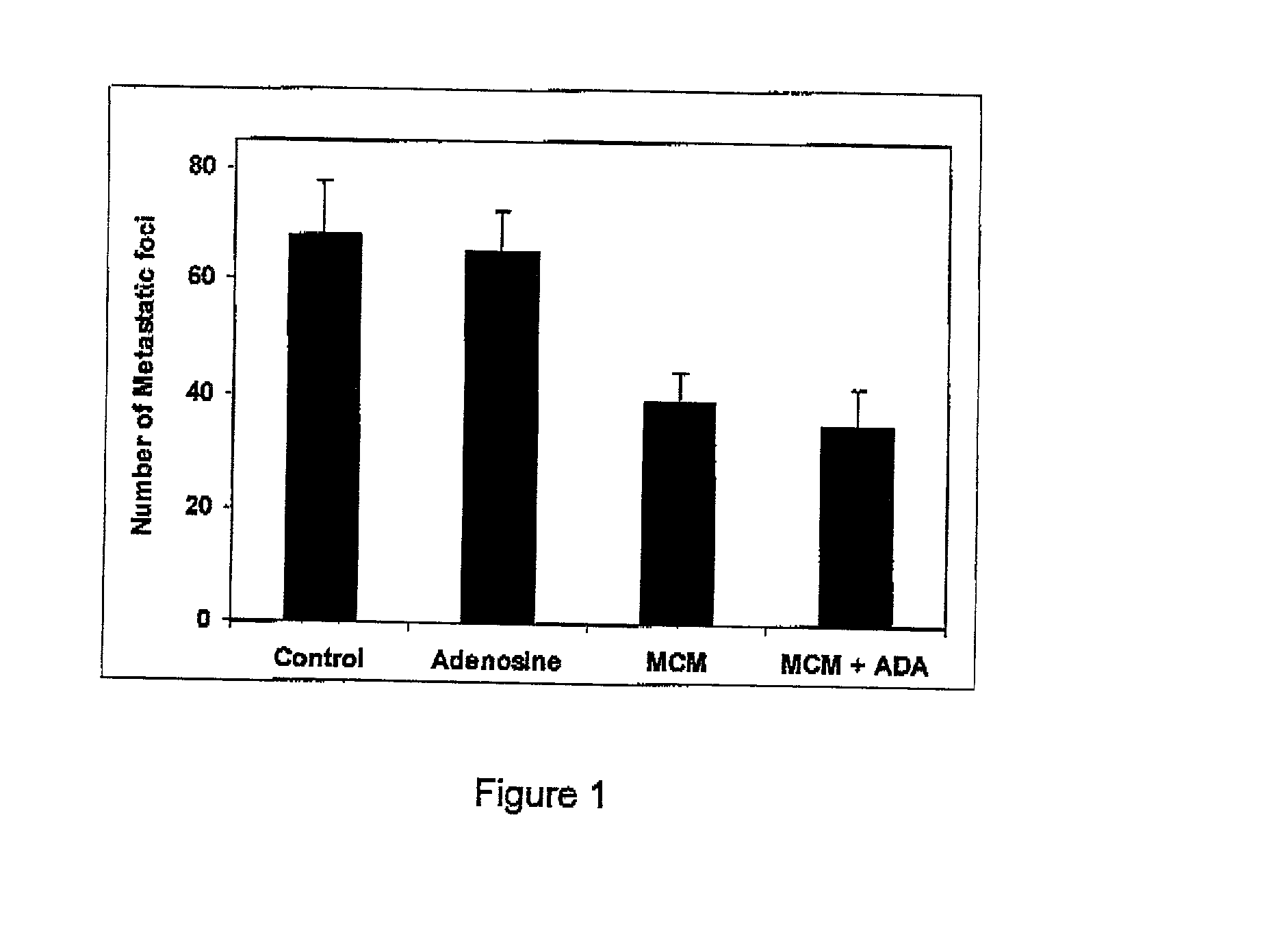
Adenosine deaminase anticancer therapy
ActiveUS8741283B2Lower Level RequirementsInhibit growthBacteriaSugar derivativesNeoplasmPharmacology
Owner:UNIKERIS LTD
Electroluminescence logic gate adopting adenosine monophosphate and adenosine deaminase as excimers
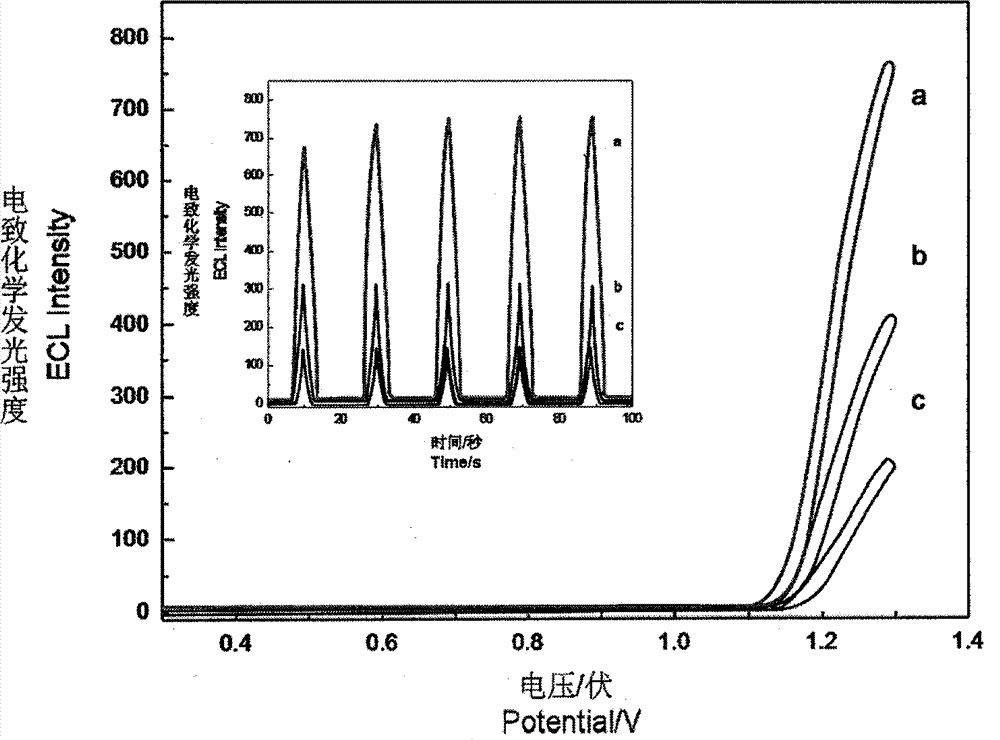
The invention relates to electrochemiluminescence DNA logic gate constructed by adopting adenosine monophosphate and adenosine deaminase as excimers based on a specific binding effect of a aptamer and a ligand thereof, and a deamination effect of adenosine deaminase, wherein adenosine monophosphate aptamer-containing single-stranded DNA and two single-stranded DNA modified with a luminescence agent and a quenching agent are subjected to hybridization to form the DNA logic gate. According to the present invention, after adenosine monophosphate and the aptamer thereof are subjected to specific binding, the luminescence agent-modified single-stranded DNA is released, and is captured by the carbon nanotube-modified electrode so as to enhance the electrochemiluminescence signal; adenosine deaminase is added to catalyze deamination of adenosine monophosphate so as to destruct the binding effect of the adenosine monophosphate and the aptamer, and the aptamer and the luminescence agent-modified single-stranded DNA form the hybrid so as to separate from the electrode and reduce the electrochemiluminescence signal; and through the improvement of the logic gate structure and the introduction of the quenching agent, the background interference is reduced, and the detection sensitivity is improved.
Owner:LINYI UNIVERSITY
Application of novel base conversion editing system on gene therapy
ActiveCN110835632AGuaranteed to workAchieve changeHydrolasesGenetic material ingredientsCytosine deaminaseBase J
The invention innovatively provides an application of a novel base conversion editing system on gene therapy for the first time; according to the system, the function of a single-base gene editing system is reserved, which is characterized in that conversion from C / G to T / A of a single base at a specified site is realized, conversion from A / T to G / C is realized, and the conversion from C / G to T / Aand from A / T to G / C can be also realized. The basic group conversion editing system comprises sgRNA, nuclease, cytosine deaminase, adenosine deaminase and uracil glycosidase inhibitors, and the nuclease, the cytosine deaminase, the adenosine deaminase and the uracil glycosidase inhibitors can target and recognize DNA sequences. The invention further provides a pharmaceutical composition containingthe base conversion editing system and the application of the pharmaceutical composition in gene editing, gene therapy, drug screening and model animal construction.
Owner:EAST CHINA NORMAL UNIV
Determination method for S-adenosylmethionine methyltransferase and kit thereof
ActiveCN102703576AEliminate distractionsEfficient responseMicrobiological testing/measurementS-Adenosyl methionineS-Adenosyl-l-methionine
The invention discloses a determination method for S-adenosylmethionine methyltransferase and a kit thereof. The determination method for S-adenosylmethionine methyltransferase comprises the step of detecting SAM methyltransferase through the enzyme coupling reaction between S-adenosylhomocysteine hydrolase and adenosine deaminase. The detection technique relieves feedback inhibition of products,has stable results and accurate quantitative reaction, and effectively overcomes the problem of radioactive pollution of the radioactive marking method. With double reagents in the kit, detection canbe carried out manually or through a full automatic biochemical analyzer within the visible range, and therefore the use and the fast detection of a plurality of samples are convenient. The determination method is also suitable for detection of the enzyme coupling reaction between S-adenosylhomocysteine hydrolase and adenosine deaminase.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
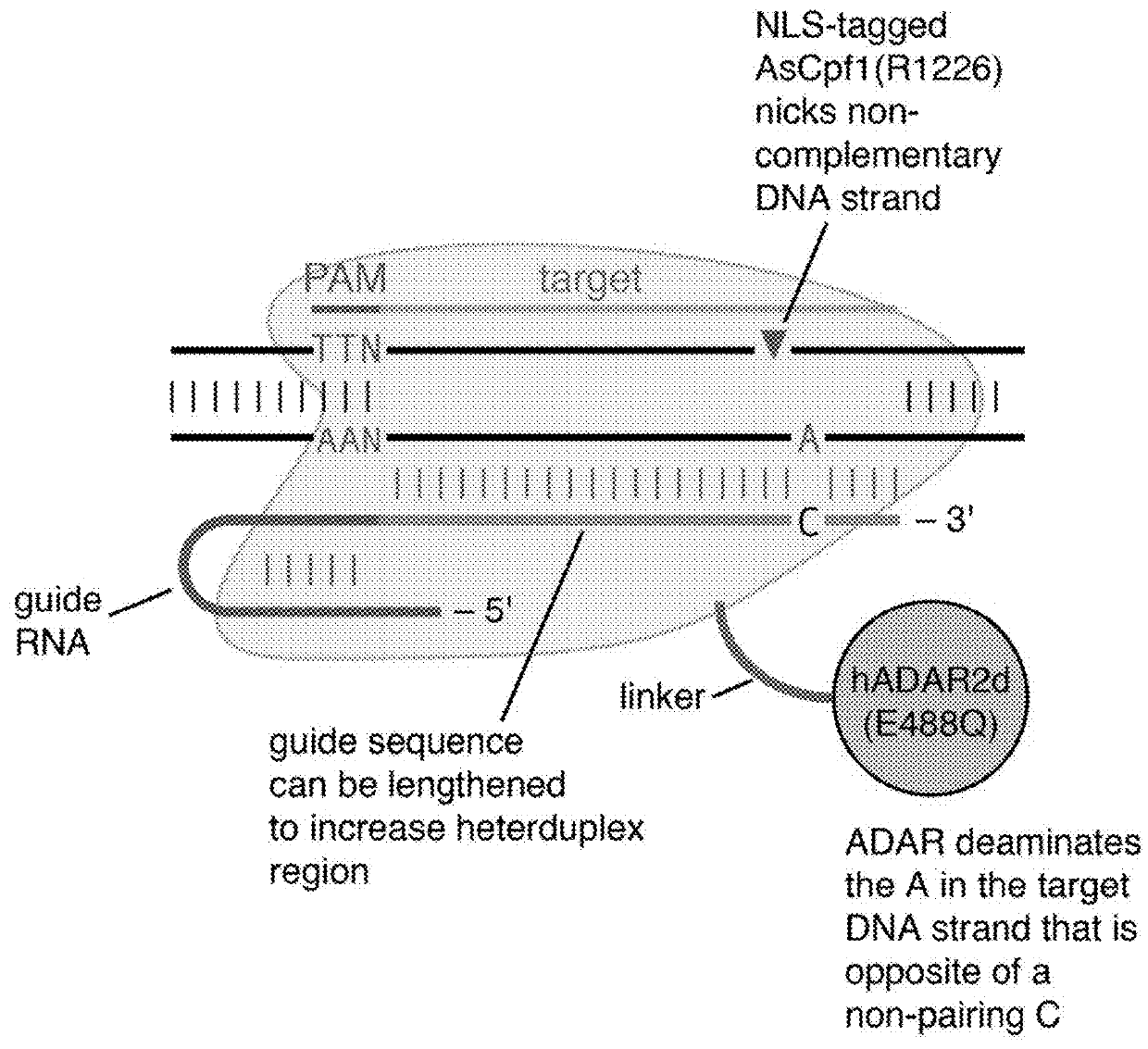
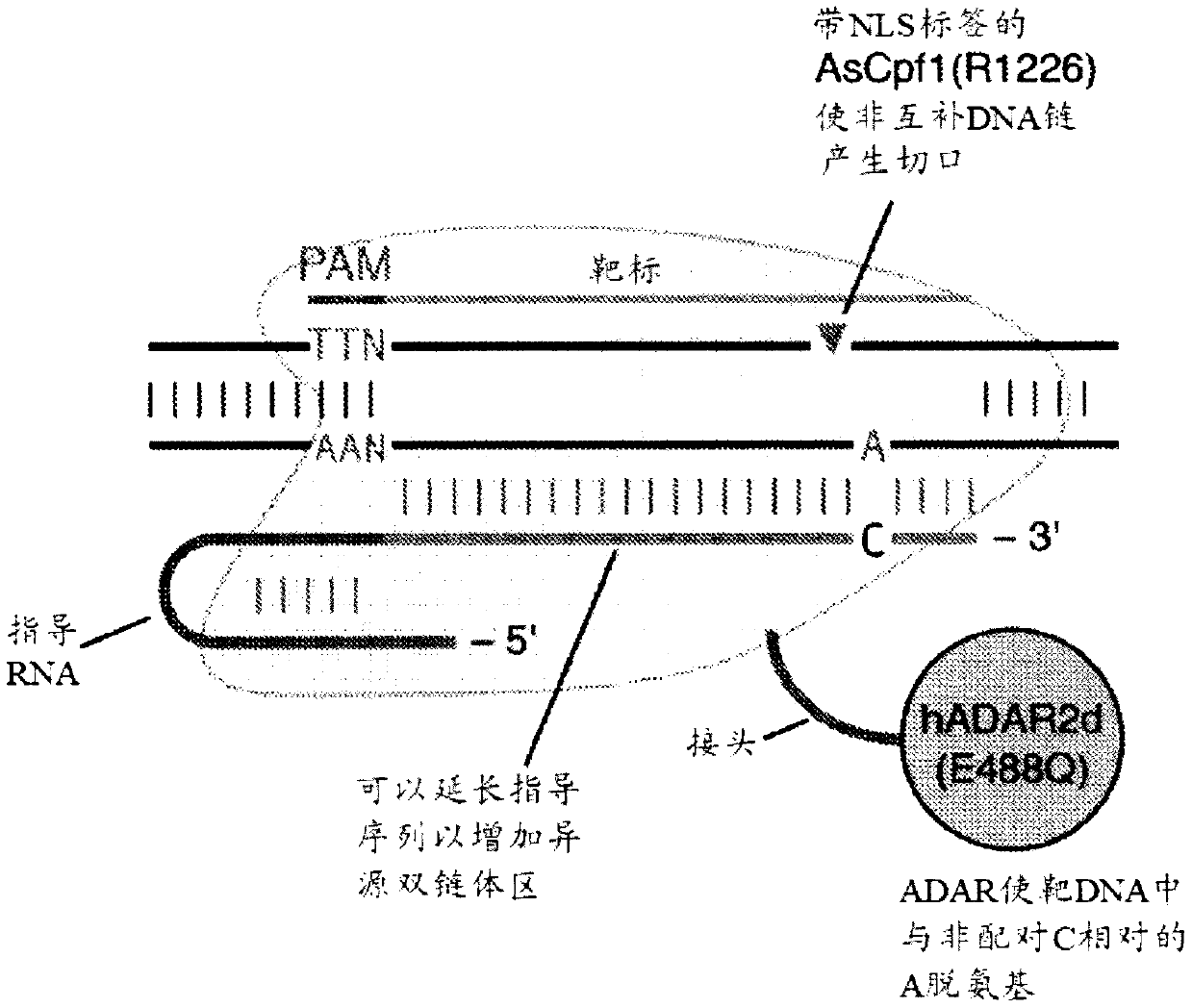
Systems, methods, and compositions for targeted nucleic acid editing
The invention provides for systems, methods, and compositions for targeting and editing nucleic acids. In particular, the invention provides non-naturally occurring or engineered DNA-targeting systems comprising a DNA-targeting Cpf1 protein, at least one guide molecule, and at least one adenosine deaminase protein or catalytic domain thereof.
Owner:THE BROAD INST INC +2
Quality control product for biochemical detection and quality control method
InactiveCN108802355AAvoid matrix effectsAvoid wastingBiological testingQuality controlDirect bilirubin
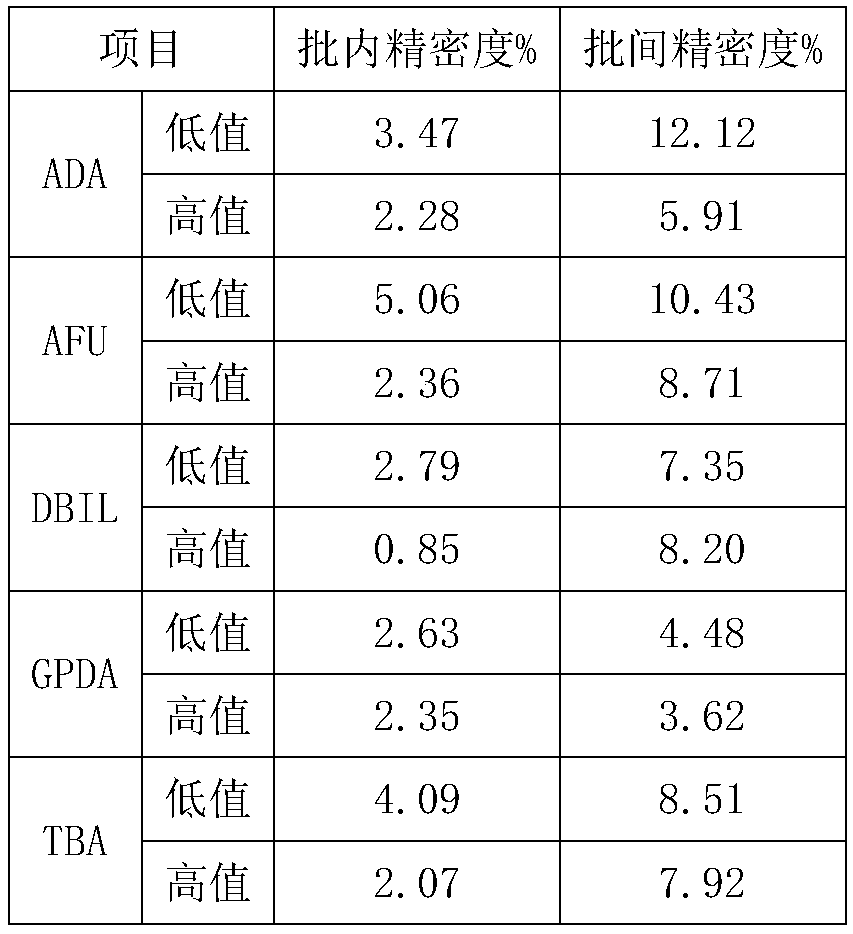
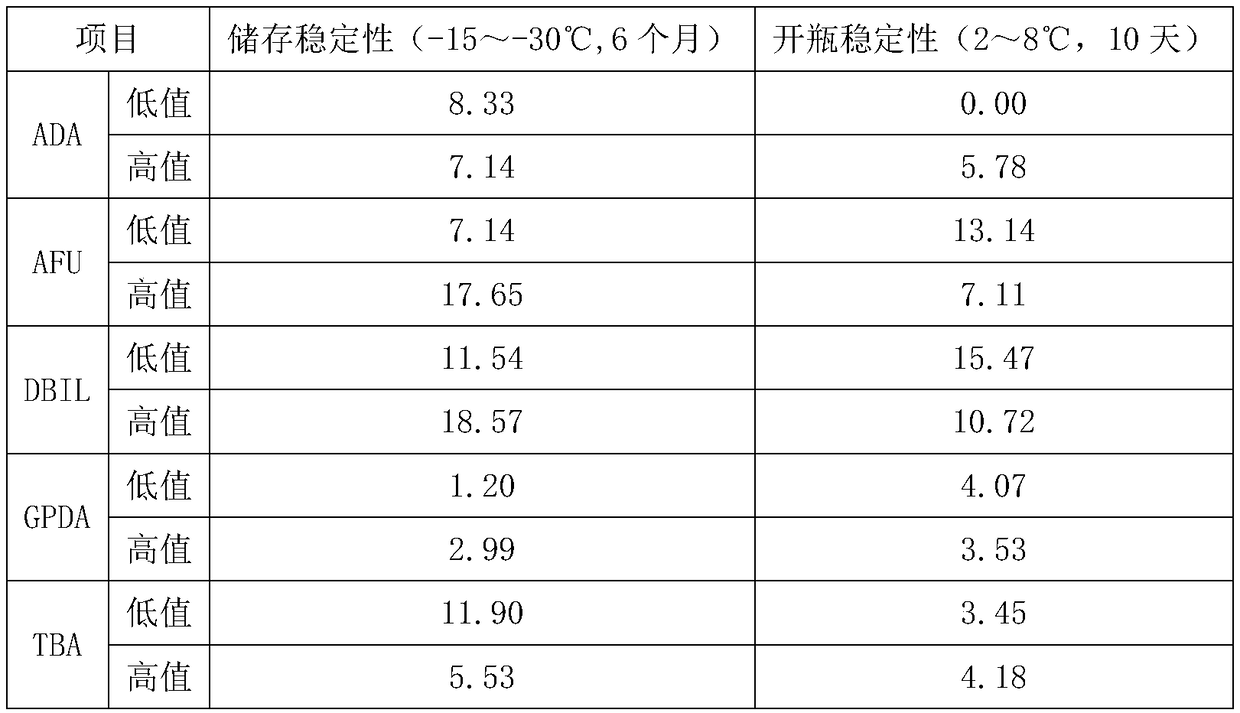
The invention relates to a quality control product for biochemical detection and a quality control method. The quality control product has the beneficial technical effects that (1) the quality controlproduct for biochemical detection is prepared by using the serum of a healthy person as the raw material, and is consistent with a clinical detection sample, so that while the matrix effect is furthest avoided, the qualifies of the five biochemical detection indexes (ADA (adenosine deaminase), AFU (alpha-fucosidase), DBIL (direct bilirubin), GPDA (glycyl-proline-dipeptidyl aminopeptidase) and TBA(total bile acid)) are effectively controlled, and the defect of lack of part or all of five substances in the existing quality control product is overcome; (2) by using the serum of the healthy person as the raw material, the waste due to no use of a large amount of remaining serum in the clinical biochemical inspection is avoided, and a large amount of original wasted serum resources is saved;(3) the quality control product for biochemical detection is prepared by scientific design, so that the good within-run accuracy and between-run accuracy and the longer stability are realized under the reasonable preservation condition, and the quality control product is suitable for being applied in clinics.
Owner:ZHEJIANG PROVINCIAL PEOPLES HOSPITAL
Xanthine dehydrogenase intercepting body and application thereof
ActiveCN105985935AAvoid limited enzymatic processesSimple production processOxidoreductasesFermentationNucleotidasePhosphoric acid
The invention discloses a xanthine dehydrogenase intercepting body and an application thereof. The protein comprises xanthine dehydrogenase intercepting body small subunit, xanthine dehydrogenase intercepting body intermediate subunit, and xanthine dehydrogenase intercepting body large subunit. The affinity of xanthine dehydrogenase intercepting body is increased by 19% by comparing a wild substrate (xanthine), the conversion number is increased by 115%, the catalysis efficiency is increased by 166%, and the temperature toleration is increased by 11 DEG C. The xanthine dehydrogenase intercepting body is in favor of developing the novel application field by combining with other enzymes, and is suitable for sample detection for detecting xanthine and hypoxanthine, the xanthine dehydrogenase intercepting body can be used for detecting inorganic phosphoric acid by combining with PNP enzyme, can be used for detecting adenosine deaminase by combining with PNP, XOD and POD enzymes and detecting 5'-nucleotidase, and is suitable for industrial application. The xanthine dehydrogenase intercepting body is in favor of increasing enzyme catalysis efficiency and reducing cost, and is in favor of industrial application.
Owner:TSINGHUA UNIV +1
Process for preparing dideoxyinosine using adenosine deaminase enzyme
InactiveUS20040175804A1Improve enzyme stabilityEasy to separateBiocideSugar derivativesDeaminase activityDideoxyadenosine
A method of making didanosine (ddI) including the steps of: (a) obtaining an enzyme expressing ddA deaminase activity; (b) immobilizing the enzyme onto an insoluble support; (c) contacting the enzyme with a dideoxyadenosine (ddA) solution of at least about 4% weight volume ddA in water for a time and under conditions to produce a ddI solution; and (d) isolating the ddI from the ddI solution. Optionally, the ddI mother liquor is reused in subsequent runs to improve yield.
Owner:BRISTOL MYERS SQUIBB CO
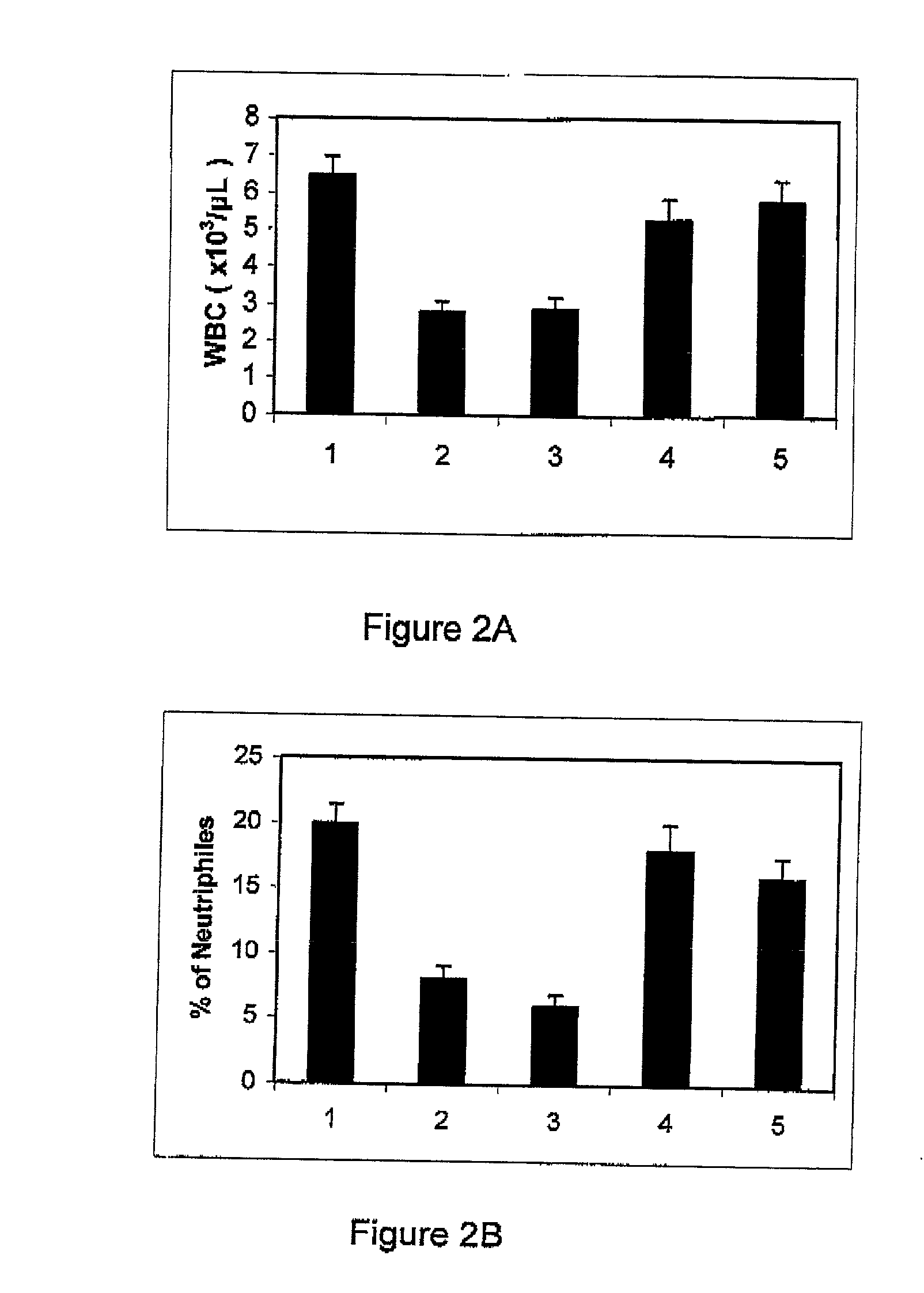
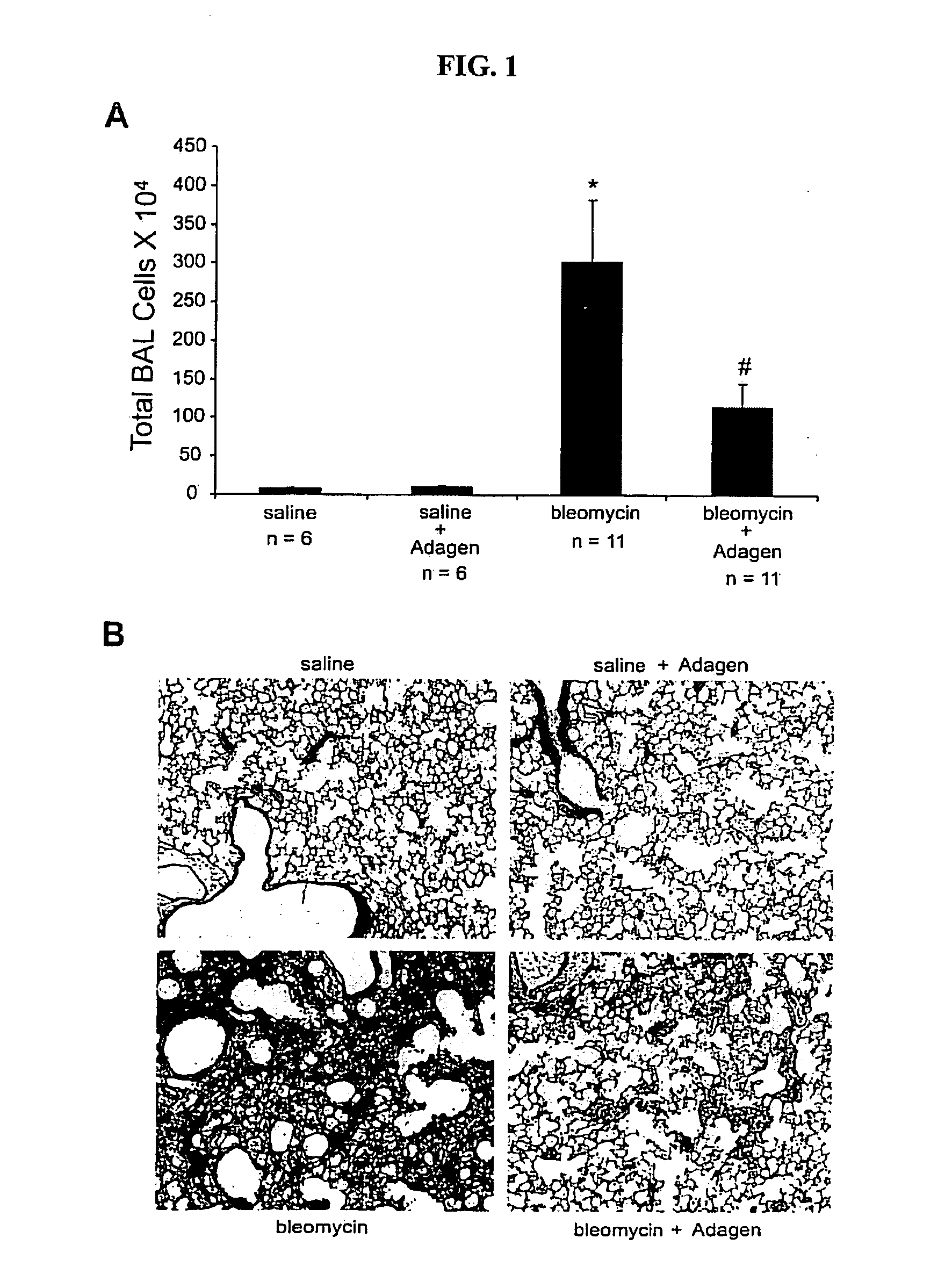
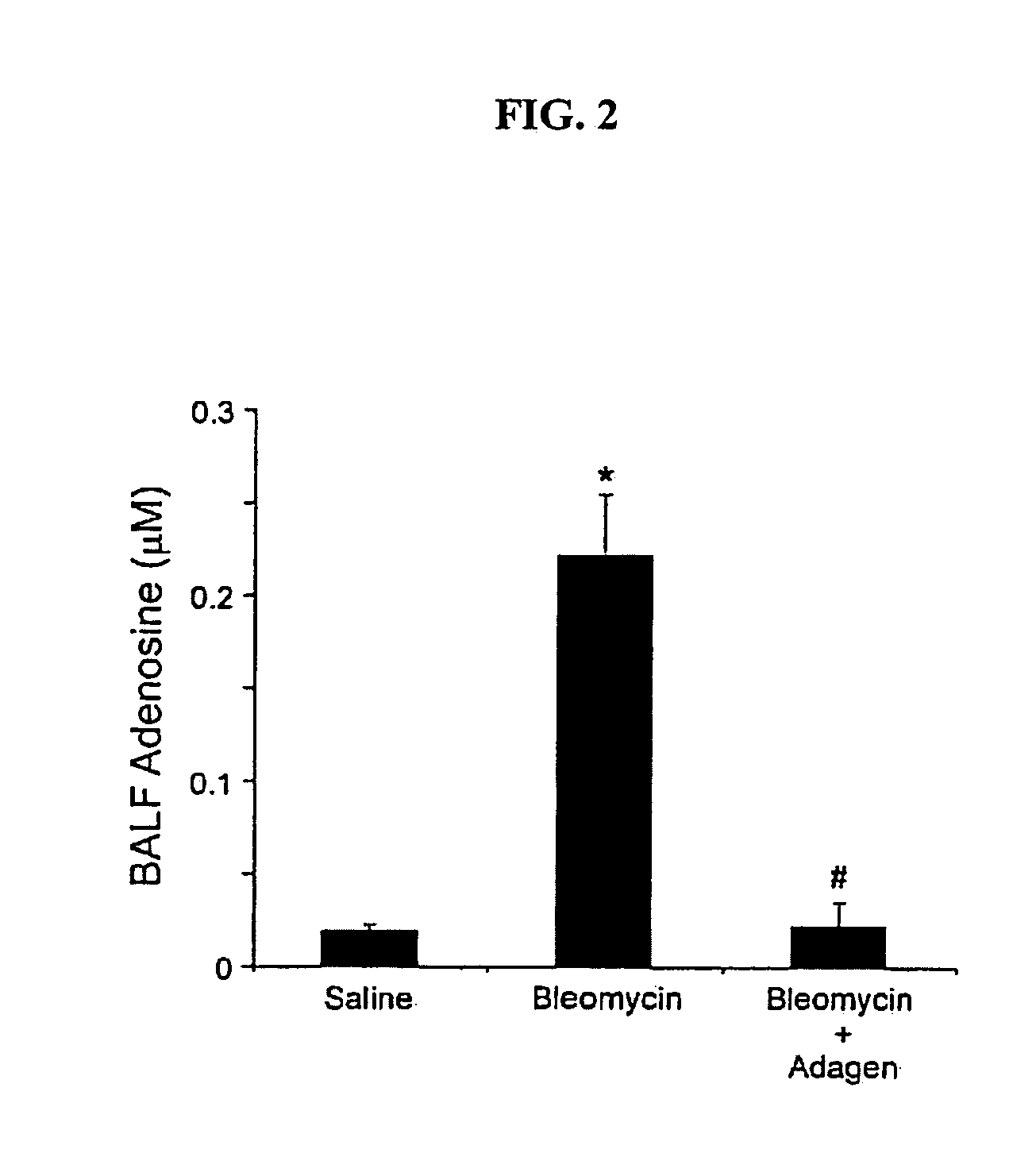
Use of adenosine deaminase for treating pulmonary disease
InactiveUS20080159964A1Powder deliveryPeptide/protein ingredientsDiseaseObstructive Pulmonary Diseases
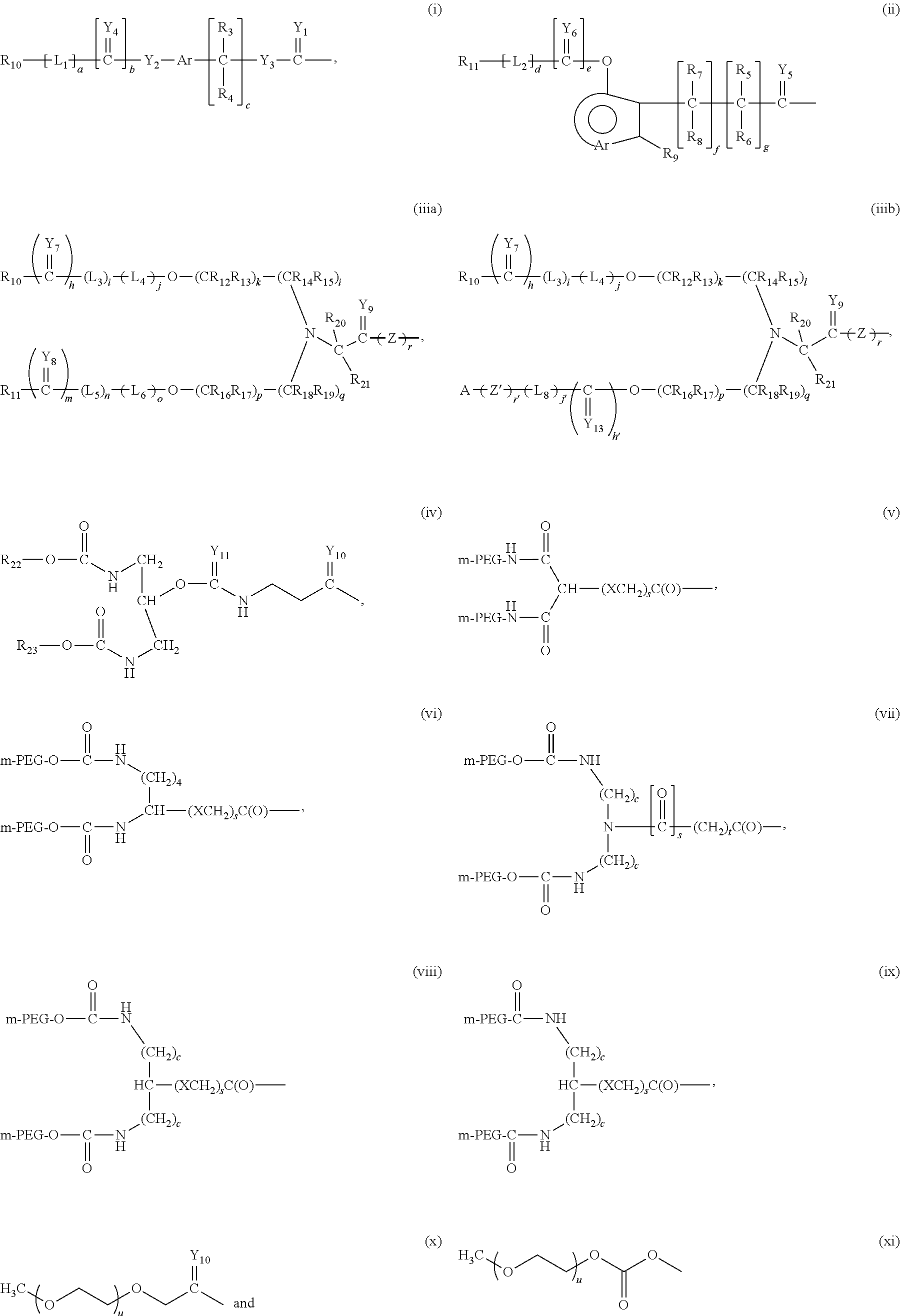
Provided are methods for treating an adenosine deaminase-mediated pulmonary disease such as asthma, pulmonary fibrosis, cystic fibrosis and chronic obstructive pulmonary disease in a mammal in need thereof, by administering and effective amount of a polymer-conjugated adenosine deaminase.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Systems, methods, and compositions for targeted nucleic acid editing
The invention provides for systems, methods, and compositions for targeting and editing nucleic acids. In particular, the invention provides non-naturally occurring or engineered DNA-targeting systemscomprising a DNA-targeting Cpfl protein, at least one guide molecule, and at least one adenosine deaminase protein or catalytic domain thereof.
Owner:THE BROAD INST INC +2
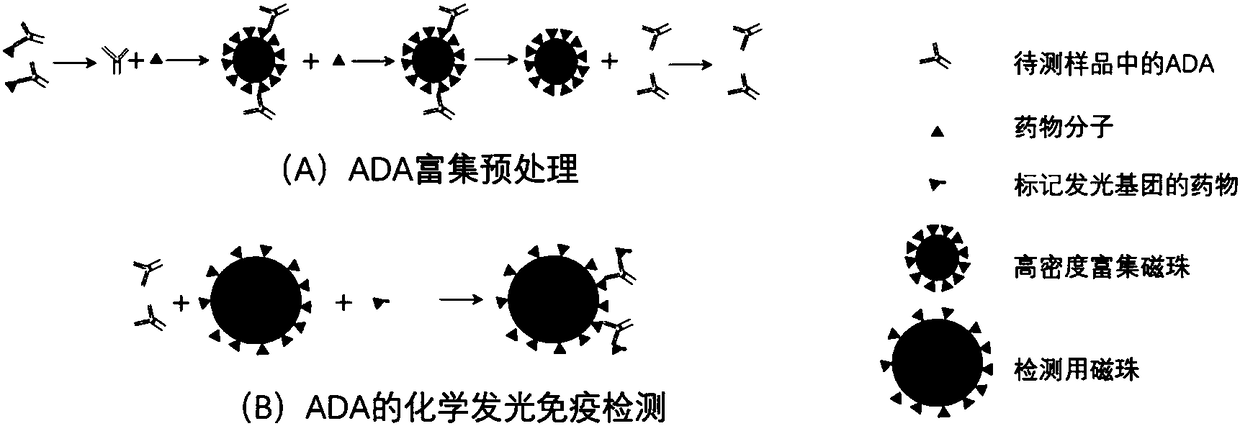
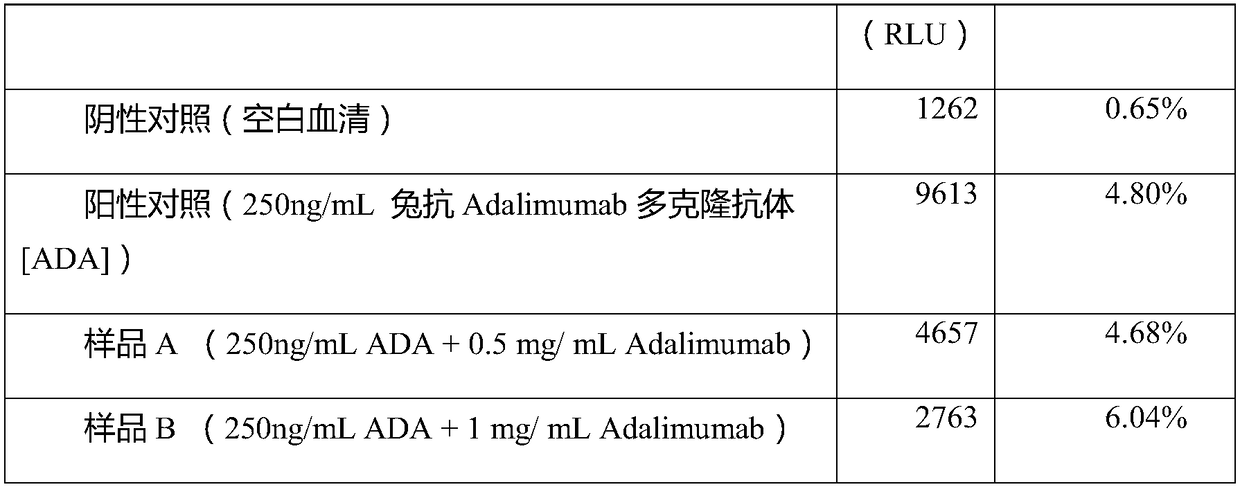
Detection method of anti-drug antibody and detection kit
ActiveCN108318680AImprove bindingEliminate distractionsBiological material analysisBiological testingHigh concentrationMagnetic bead
The invention discloses a detection method of an anti-drug antibody and a detection kit. The detection method comprises the following steps of: pretreating a sample to be detected and detecting the pretreated sample, and after high-density enriching magnetic beads are adopted for enriching the sample to be detected, chemiluminesent immunoassay of magnetic particles is carried out on the sample. The detection method and the detection kit disclosed by the invention have the beneficial effects that by pretreatment from the high-density enriching magnetic beads, the detection for the total ADA (Adenosine Deaminase) existing in the form of drug-ADA complex in the sample can be realized, so that the influence of high-concentration drug protein in the sample can be overcome, and the drug resistance of the ADA detection method can be improved; simultaneously the detection method also has the advantages of fast and automatic analysis of a chemiluminesent immunoassay method. The detection kit comprises a high-density enriching magnetic bead reagent, a magnetic bead reagent for detection, a drug for labeling luminophores, sample diluted liquid, acidifying liquid, neutralizing liquid, a quality control product and exciting liquid. By adoption of the kit, the anti-drug antibodies can be rapidly and accurately detected.
Owner:北京新艾进生物科技有限公司
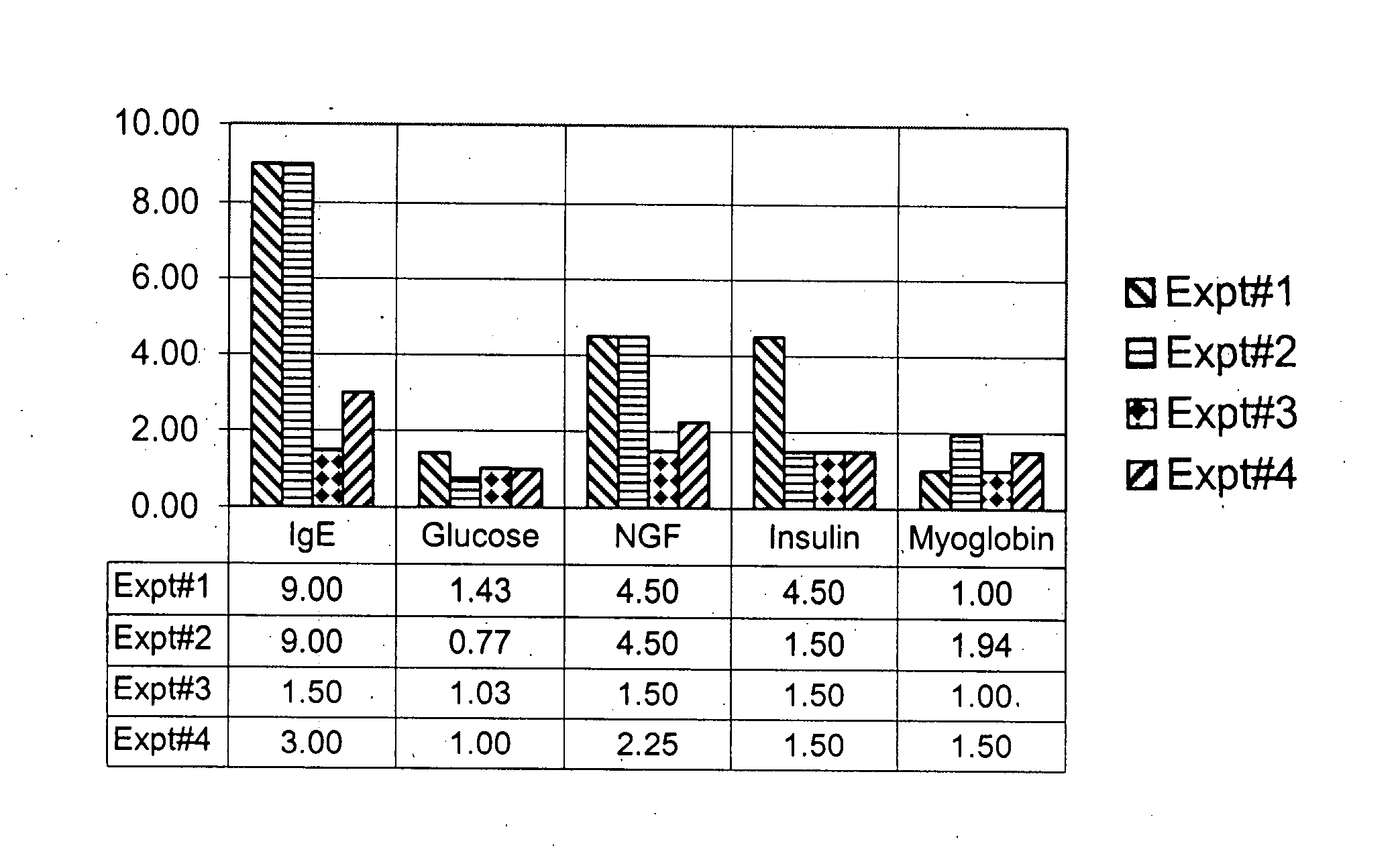
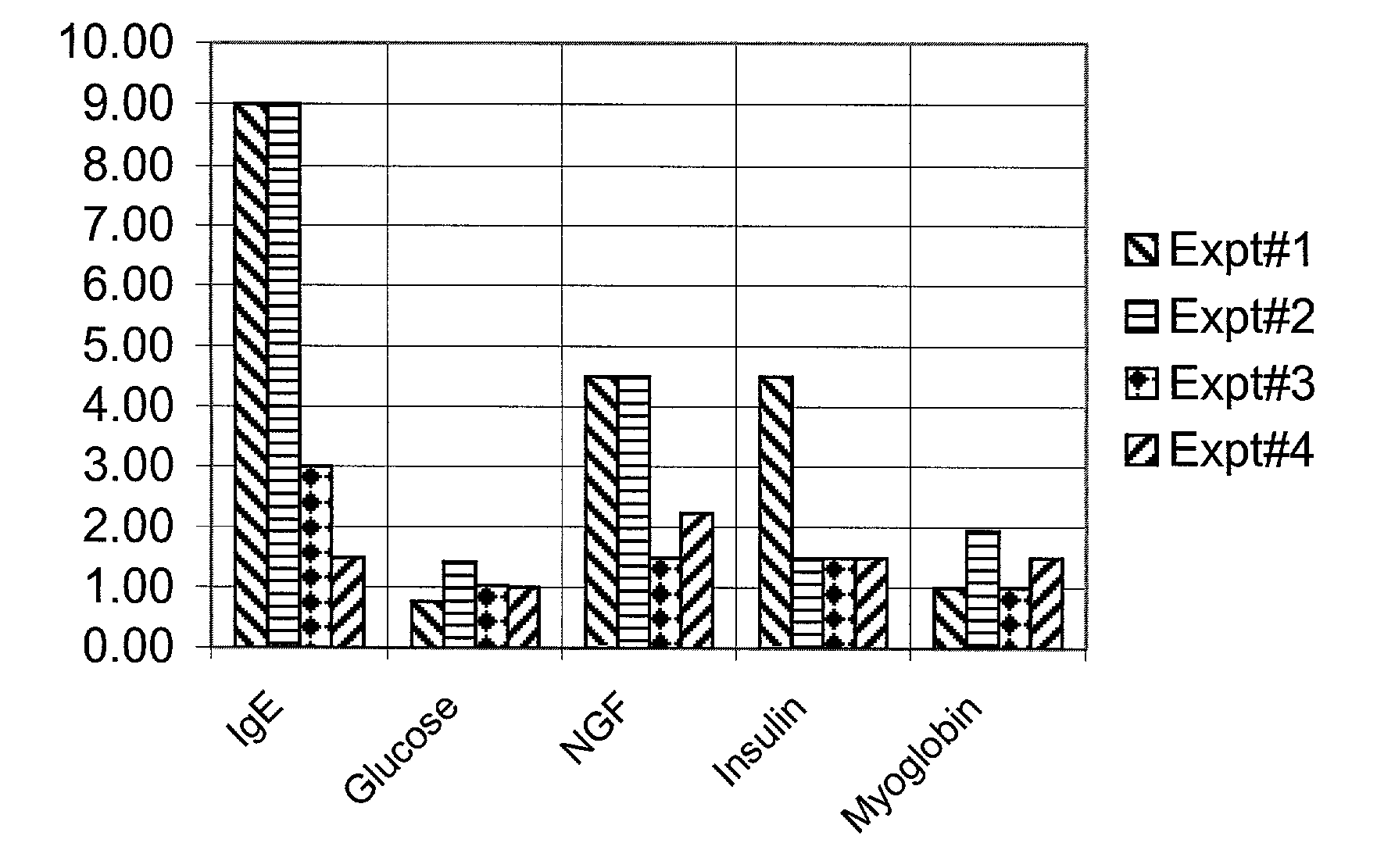
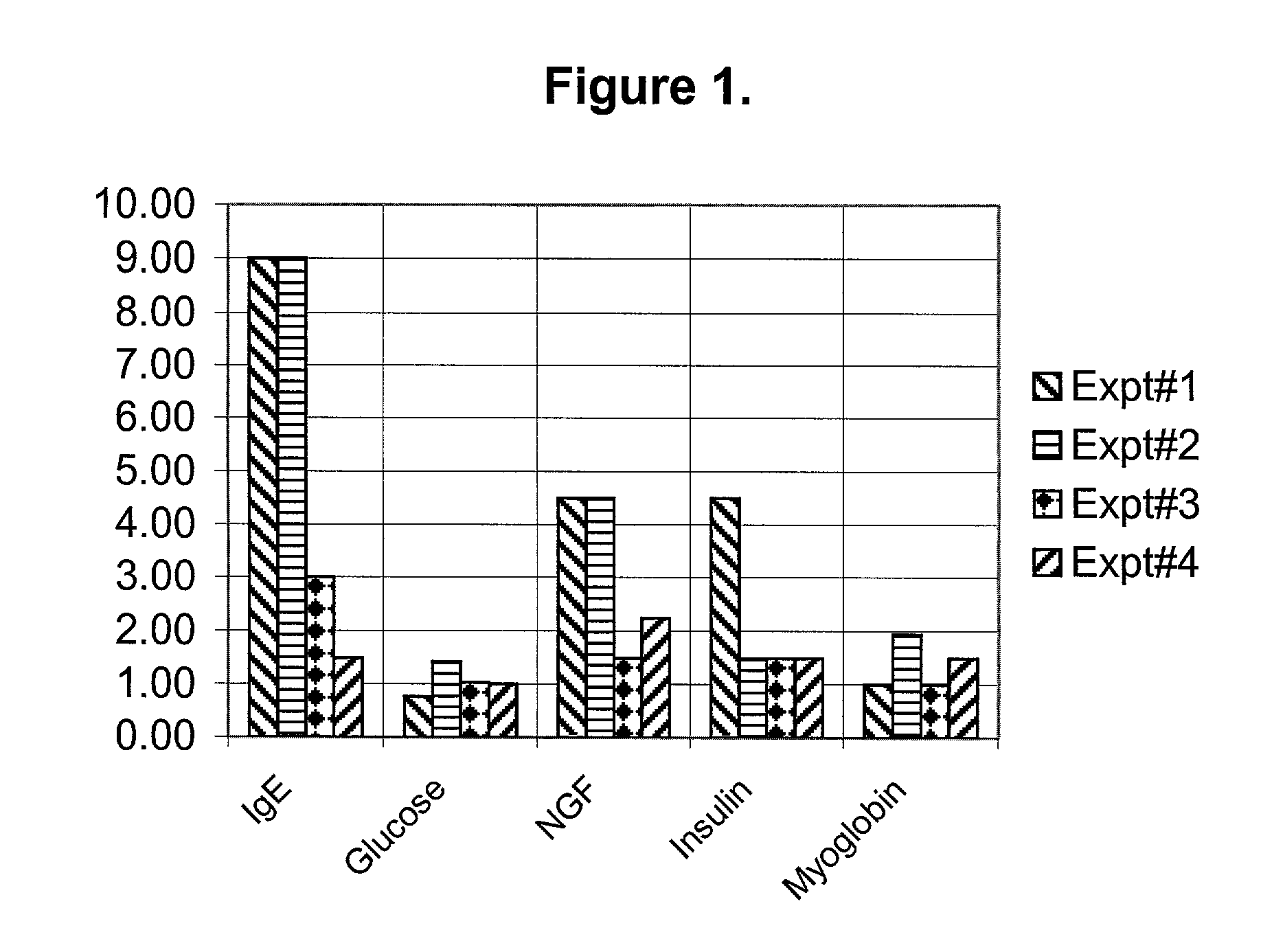
Diagnosis and treatment for immunoglobulin E (IgE) implicated disorders
InactiveUS20080081788A1Low serum levelsLowering free IgE in serumPeptide/protein ingredientsMetabolism disorderAutoimmune conditionInsulin dependent
Human saliva is used as a non-invasive source instead of invasive blood serum plasma for detection and assay of endogenously present proteins; nerve growth factor (NGF), myoglobin, Insulin, adenosine deaminase (ADA), including immunoglobulin E (IgE). It was discovered that people having high levels of IgE, show high levels in comparison to the normal controls of NGF, myoglobin, insulin and ADA, disrupting the homeostasis for these proteins. Oral administration of a synthetic peptide LT-10 disclosed in U.S. Pat. No. 5,576,297 having sequence L K A M D P T P P L reduces free IgE level in humans and brings other proteins int homeostasis, for example, NGF, myoglobin, insulin and ADA and possibly other proteins and cytokines. Composition of synthetic LT-10 is advocated as a treatment for IgE implicated disorders such as asthma, depression and various types of autoimmune diseases, such as erythematosus (SLE); Rheumatoid arthritis Sjogren's syndrome; Reiter's syndrome; Diabetes mellitus (insulin-dependent); Graves' disease; Addison's disease; Hodgkin's disease, etc.
Owner:LIPPS BINIE V
Stable cyclic enzymatic detection kit for homocysteine
ActiveCN110308282AQuick checkWide linear rangeBiological material analysisBiological testingS-Adenosyl-l-methionineAntioxidant
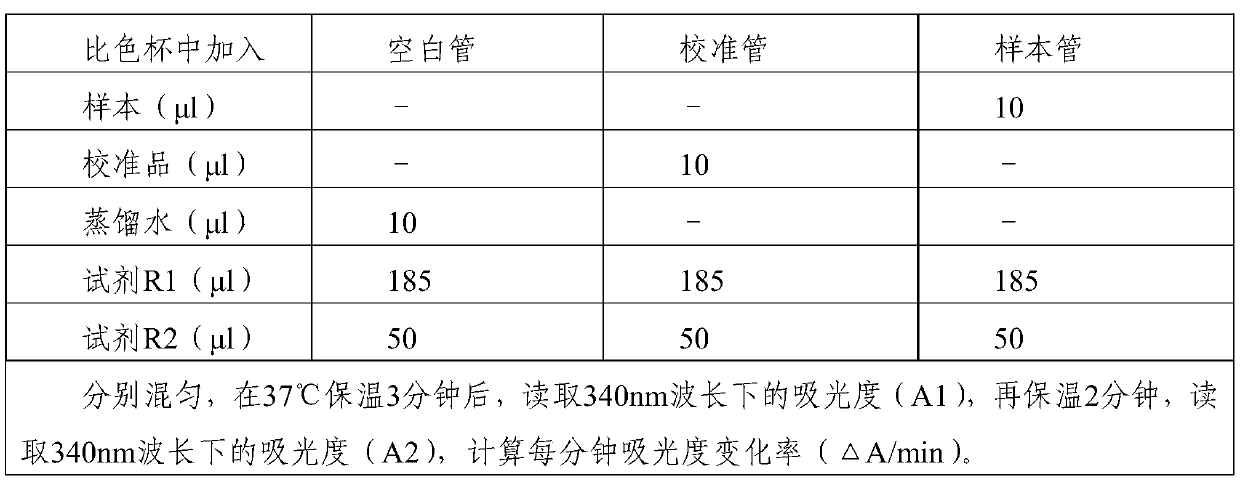
The invention provides a stable cyclic enzymatic detection kit for homocysteine. The kit comprises reagents R1 and R2. The reagent R1 is composed of a buffer solution, a surfactant, a protective agent, an antioxidant, ethylene diamine tetraacetic acid, tris(2-carbonylethyl) phosphine hydrochloride, S-adenosyl methionine, 2-ketoglutaric acid, a reduced coenzyme, and a preservative. The reagent R2 includes a buffer solution, a surfactant, a protective agent, S-adenosyl-L-homocysteine hydrolase, Hcy transmethylase, adenosine deaminase, glutamate dehydrogenase, and a preservative. According to thekit, because of the cooperative effect of the surfactants, the protective agents and the antioxidants, the provided kit has advantages of high precision, high sensitivity, wide linear range and highstability and the like by being compared with the commercially available HCY kit, thereby providing the important base for the auxiliary diagnosis of clinical HCY related diseases.
Owner:BIOSINO BIO TECH & SCI
Stem cell culture medium and preparation method thereof
InactiveCN106635957AShorten the timeEliminate pollutionCulture processVertebrate cellsCell culture mediaStem cell culture
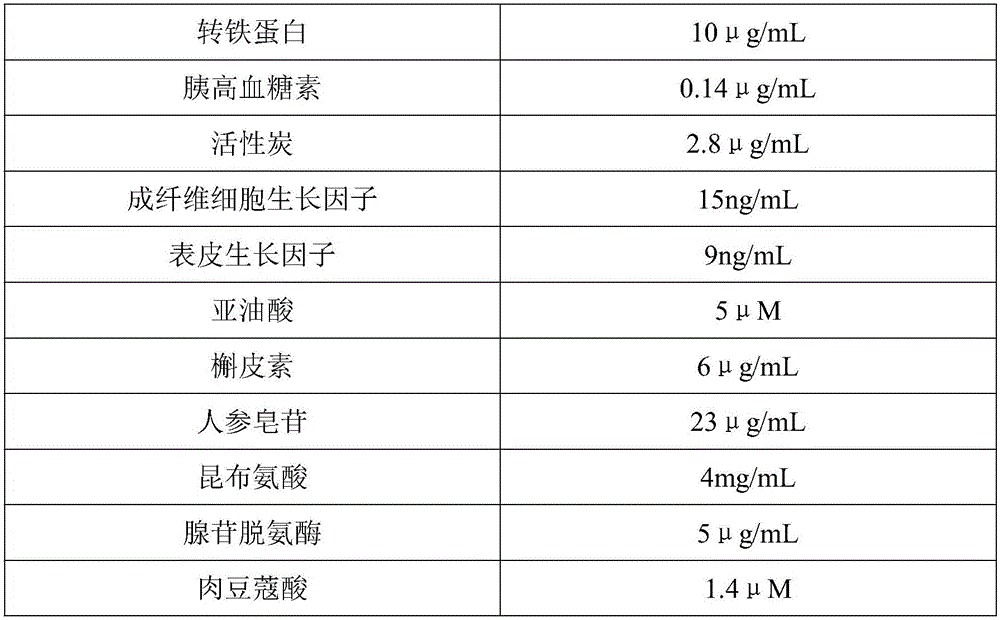
The invention belongs to the technical field of cell culture mediums, and particularly relates to a stem cell culture medium and a preparation method thereof. The stem cell culture medium provided by the invention comprises a basic culture medium and an additive, wherein counted by final concentration, the additive is prepared from 1 to 3mg / mL human serum albumin, 8 to 13mug / mL transferrin, 0.12 to 0.15mug / mL glucagon, 2.5 to 3mug / mL activated carbon, 13 to 18ng / mL fibroblast growth factor, 7 to 11ng / mL epidermal growth factor, 3 to 6 muM linoleic acid, 5 to 9mug / mL quercetin, 20 to 25mug / mL platycodin, 2 to 5mg / mL laminine, 3 to 7mug / mL adenosine deaminase and 1.2 to 1.6muM myristic acid. By adopting the stem cell culture medium provided by the invention, stem cells can be amplified rapidly, and the culture time of the stem cells is shortened greatly.
Owner:叶宗耀
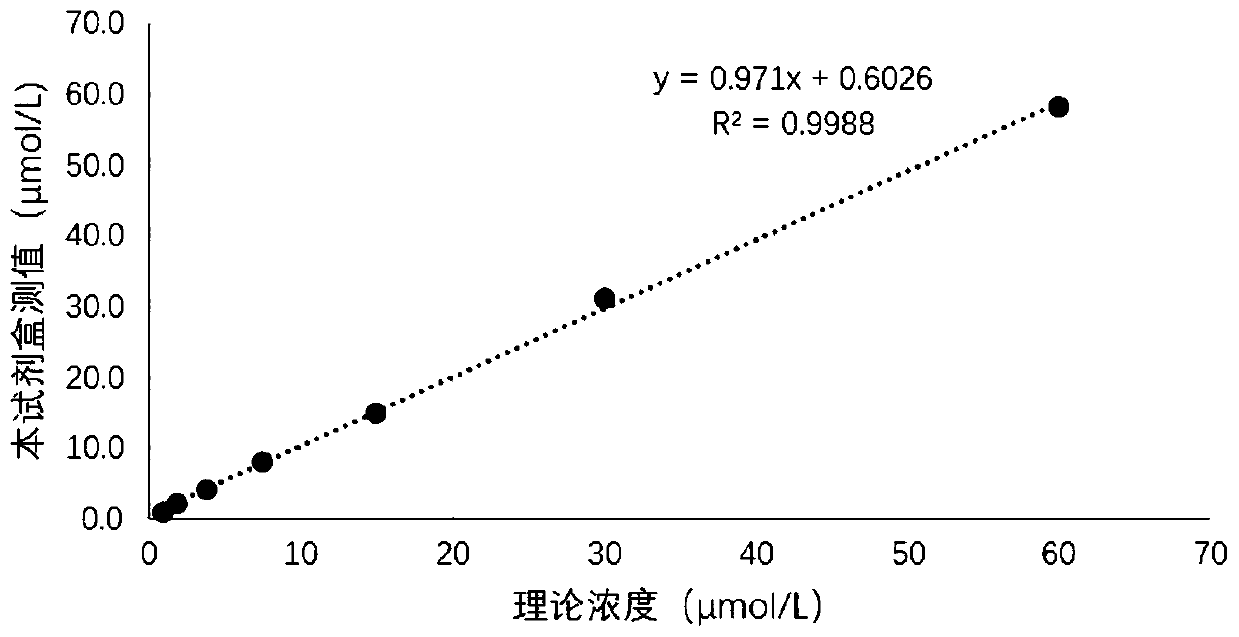
Stable and high-interference resistance serum adenosine deaminase detection reagent and detection method
InactiveCN106119339AAvoid interferenceImprove anti-interference abilityMicrobiological testing/measurementPolyethylene glycolAscorbate Oxidase
The invention relates to the technical field of detection of serum adenosine deaminase and in particular relates to a serum adenosine deaminase detection reagent. R1 comprises buffer solution, 4-aminoantipyrine, PNP, XOD, POD, bilirubin oxidase, ascorbic acid oxidase, glycerin, polyethylene glycol 6000, ethylene glycol, mannitol, mycose, BSA, alkyl glycoside and preservatives; R2 comprises buffer solution, adenosine, 3-hydroxy-2,4,6-triiodobenzoic acid, glycerin, polyethylene glycol 6000, ethylene glycol, mannitol, mycose, BSA, alkyl glycoside and preservatives. With the adoption of the novel Trinder reaction chromogen substance 3-hydroxy-2,4,6-triiodobenzoic acid, multiple stabilizers are added, and the stability of the reagent is obviously improved; and due to the added bilirubin oxidase, ascorbic acid oxidase and novel nonionic surfactant alkyl glycoside (APG), interference of bilirubin and ascorbic acid is avoided, a turbid reaction system is prevented, and the substrate stability and the interference resistance of the reagent are enhanced.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Serum-free medium for stem cells
ActiveCN104694470AMaintain biological propertiesMaintenance of multilineage potentialSkeletal/connective tissue cellsBlood/immune system cellsSerum free mediaStem cell culture
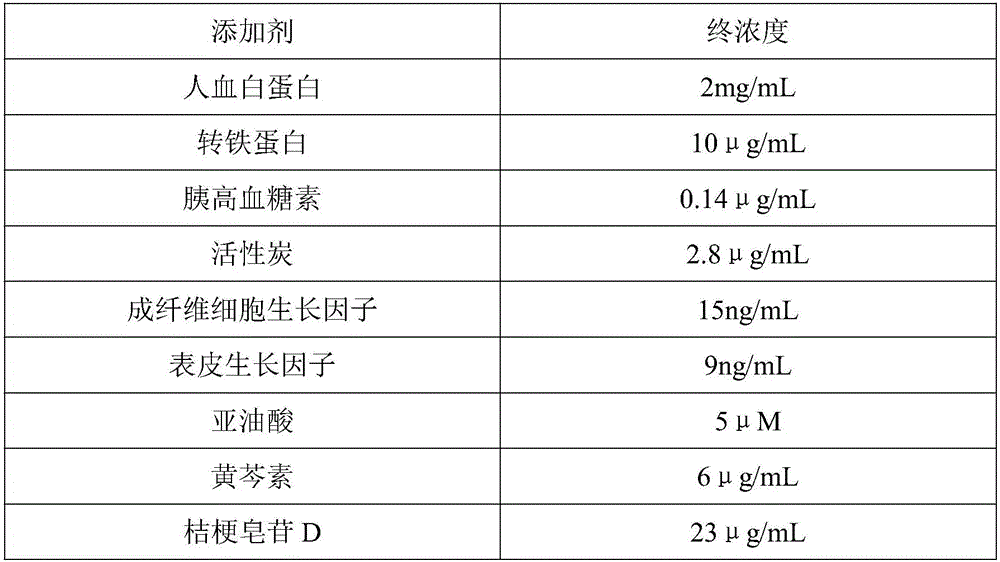
The invention relates to the biological field, in particular to a serum-free medium for stem cells. A medium additive comprises 5-30 g / L of human serum albumin, 1-10 g / L of plant-derived recombinant human serum transferring, 0.1-8 g / L of alpha1 acid glycoprotein, 0.1-8 g / L of alpha2-HS-glycoprotein, 0.05-5 g / L of apolipoprotein AI, 0.05-5 g / L of apolipoprotein AII, 0.01-2 g / L of hemopexin, 0.05-5 g / L of adenosine deaminase, 0.03-3 g / L of serine protease inhibitors, 0.01-3 g / L of transthyretin and 0.01-3 g / L of human insulin. According to the serum-free medium for stem cells, pathogen pollution from animal origins is eliminated, the components are defined, and therefore reproducibility and controllability of the serum-free medium are better than those of a serum medium; the effect of in-vitro culture of stem cells is superior to the effect of the serum medium, the expansion speed of stem cells is increased, and culture time for stem cells is greatly shortened.
Owner:JIANGSU PURECELL BIOMEDICAL TECH CO LTD
Method for determining stability of diagnostic reagent of adenosine deaminase by improving coupling enzymatic reaction
InactiveCN101514358AReduce wasteImprove stabilityMicrobiological testing/measurementBovine serum albuminStabilizing Agents
The invention provides a method for determining the stability of a diagnostic reagent of adenosine deaminase by improving a coupling enzymatic reaction, which comprises the steps of adding different stabilizing agents in the diagnostic reagent of the adenosine deaminase, wherein the stabilizing agents comprise 10 to 100 mmol / L of calcium ethylene diamine tetracetate, 10 to 100 mmol / L of ferric ethylene diamine tetracetate, 5 to 100 mmol / L of sodium molybdate, 5 to 100 mmol / L of ammonium molybdate, 5 to 100 mmol / L of sodium glutamate, 8 to 10g / L of bovine serum albumin, and 0.5 to 10.0 KU / L of superoxide dismutase. A coupling enzymatic reaction reagent for determining the adenosine deaminase has higher stability by adding any one of the stabilizing agents into the reagent and maintaining the stabilizing agents within the concentration range; the method, which can be widely applied to clinical in vitro diagnosis in vitro and reduces the waste of the reagents.
Owner:AILEX TECH GRP CO LTD
Stable adenosine deaminase reagent high in anti-interference capability and detection method
InactiveCN105238847AImprove stabilitySuitable for automatic analysisMicrobiological testing/measurementSucrosePeroxidase
The invention relates to the technical field of adenosine deaminase detection, in particular to an adenosine deaminase detection reagent. A reagent R1 comprises a buffer solution, 4-aminoantipyrine, BSA, cane sugar, trehalose, peroxidase, ascorbic acid oxidase, bilirubin oxidase and preservatives; a reagent R2 comprises a buffer solution, adenosine, EHSPT, BSA, cane sugar and preservatives. The adenosine deaminase detection reagent has the advantages that the phosphate buffer solution is adopted, the stabilizer BSA, the cane sugar and the trehalose are added, and accordingly, stability of the reagent is improved greatly; determination performance is improved remarkably, and stability and anti-interference capability of the reagent are enhanced.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Method and kit for investigating adenosine deamiase by coupling enzymatic reaction
InactiveCN1693881ALow costSimplified inosine coupling enzymatic reaction systemMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementHydrogenPeroxidase
Heinz(1980) makes use of the adenosine ammonia-deleted enzyme to change adenosine into hypo-yellow and gains H2O2 through the reaction of the enzyme1 and enzyme2. With the effect of the catalase and aldehyde hydrogen-deleted enzyme, Heinz measures the velocity of increase of the degree of NADPH absorbing light at 334 nms to gain adenosine ammonia-deleted enzyme's activity. The method's weakness is high cost of the reagents. The invention introduces Trinder's reaction to combine aniline type hydrogen and 4- to from a coloured product with the effect of H2O2 gained by the reaction of enzyme3 in the situation of the catalase. By watching the quantity of this coloured product, people can determine the adenosine ammonia-deleted enzyme's activity. The invention simplifies the Heinz's reaction system and lowers the cost. The invention uses the aniline compound ADOS, ADPS, ALPS, TODB, TOOS and TOPS as hydrogen provider for Trinder's reaction and increases the reaction's agility. The invention also involves a reagent box used for implementing the method mentioned above.
Owner:ZHEJIANG YAKE SCI & TECH +1
Nucleotide production process
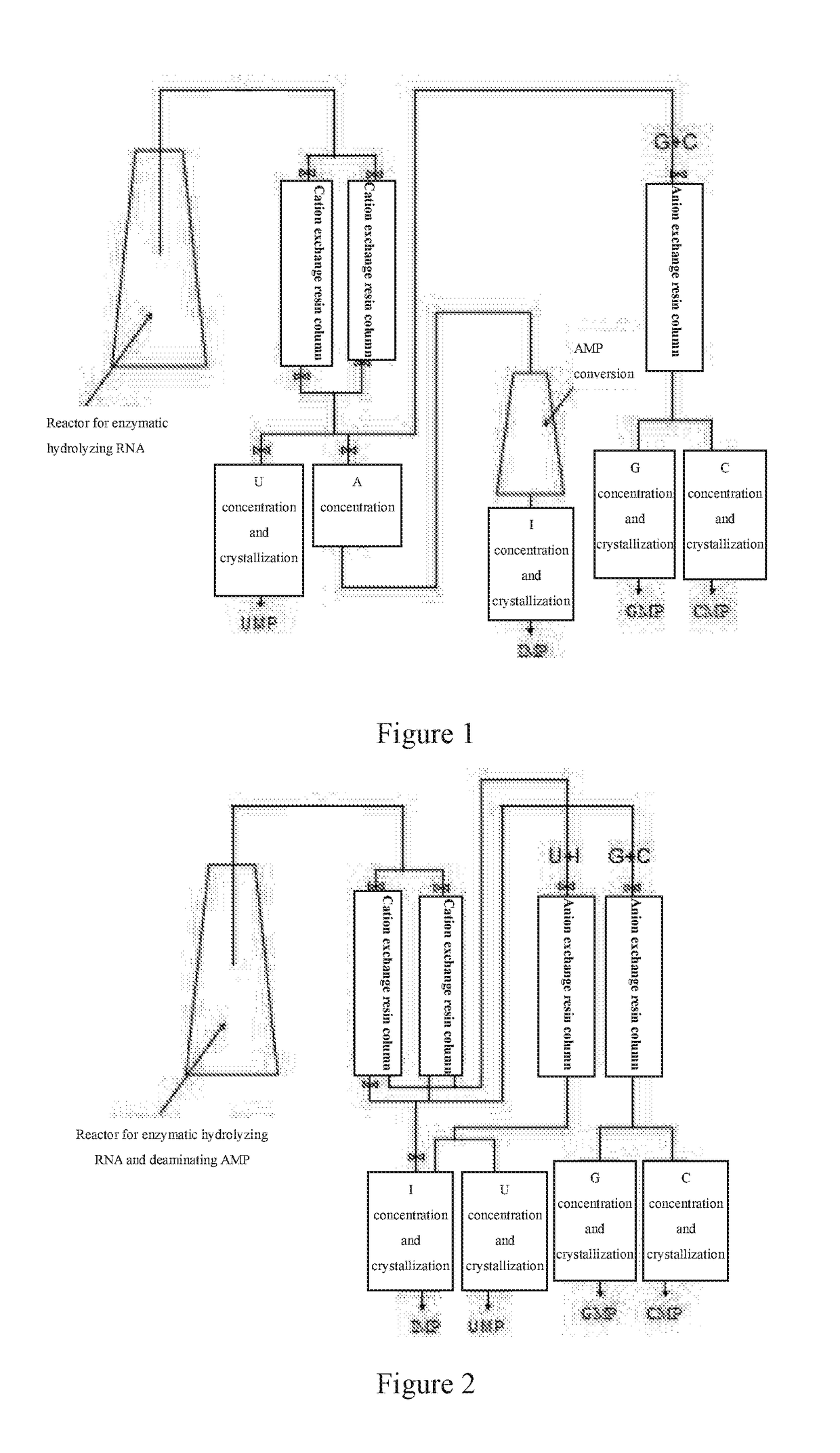
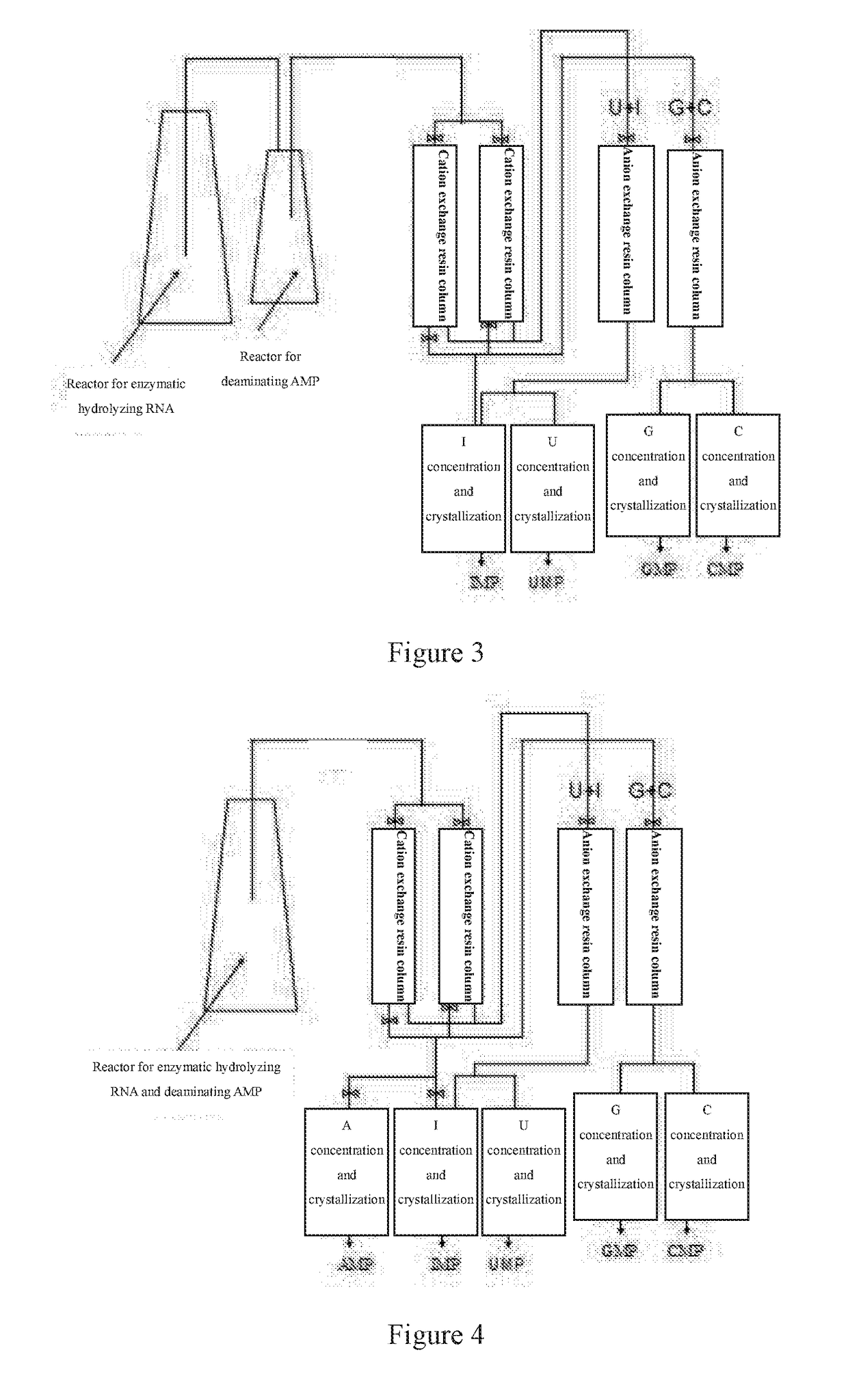
A nucleotide production process comprises: decomposing an RNA by using a nuclease P1 so as to obtain nucleotides AMP, GMP, CMP and UMP, converting part or all of the nucleotide AMP into a nucleotide IMP by using adenosine deaminase, separating the obtained nucleotide by using an ion exchange resin, and then performing concentration and crystallization to obtain purified nucleotides AMP, GMP, CMP, UMP and IMP or obtain purified nucleotides GMP, CMP, UMP and IMP. The whole biocatalysis production of nucleotides is implemented by using a double-enzyme catalysis method, and high-purity nucleotides are obtained by using an ion resin separation technology and a solvent crystallization method; and the production process is simple and environmentally-friendly, and has low costs, high product safety and purity.
Owner:NANJING UNIV OF TECH
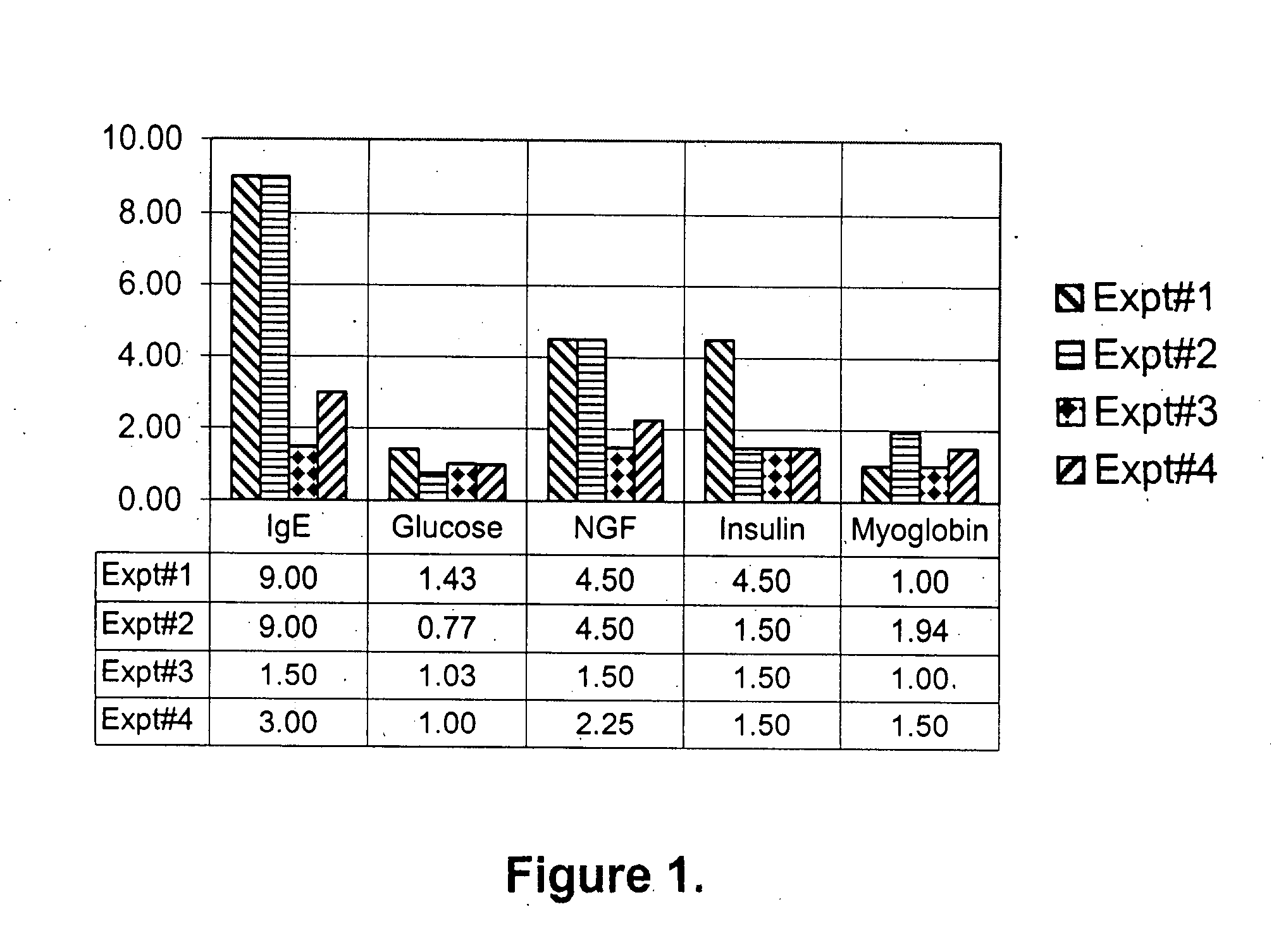
Diagnosis and treatment for immunoglobulin E ( IgE) implicated disorders
Human saliva is used as a non-invasive source instead of invasive blood serum plasma for detection and assay of endogenously present proteins; nerve growth factor (NGF), myoglobin, Insulin, adenosine deaminase (ADA), including immunoglobulin E (IgE). It was discovered that people having high levels of IgE, show high levels in comparison to the normal controls of NGF, myoglobin, insulin and ADA, disrupting the homeostasis for these proteins. Oral administration of a synthetic peptide LT-10 disclosed in U.S. Pat. No. 5,576,297 having sequence L K A M D P T P P L reduces IgE level in humans and bring other proteins into homeostasis, for example, NGF, myoglobin, insulin and ADA and possibly other proteins and cytokines. Composition of synthetic LT-10 is advocated as a treatment for IgE implicated disorders such as asthma, depression and various types of autoimmune diseases, such as erythematosus (SLE); Rheumatoid arthritis Sjogren's syndrome; Reiter's syndrome; Diabetes mellitus (insulin-dependent); Graves' disease; Addison's disease; Hodgkin's disease, etc.
Owner:LIPPS BINIE V +1
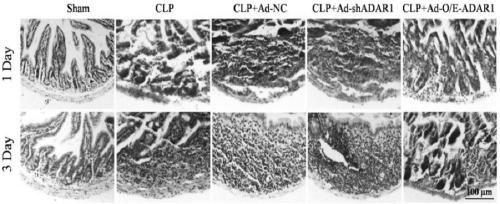
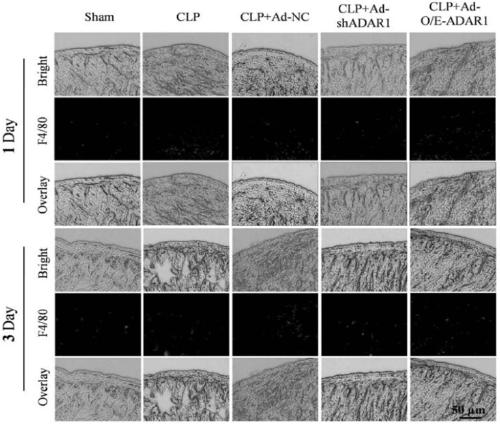
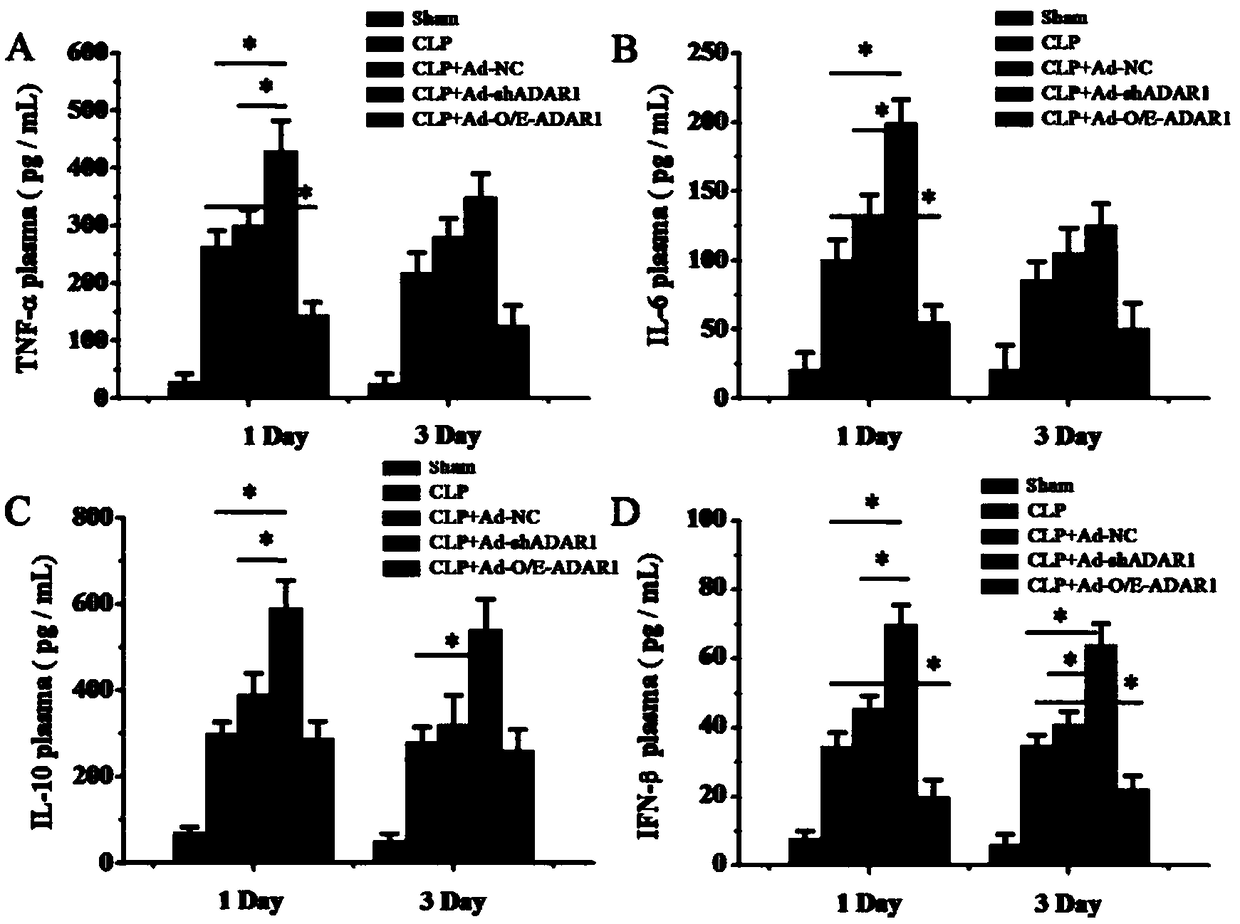
ADAR1 (Adenosine Deaminases Acting on RNA1) over-expressed virus vector as well as construction method and application thereof
The invention provides an ADAR1 over-expressed virus vector, a preparation method of the virus vector, and application of the ADAR1 over-expressed virus vector in aspects of protecting the intestinalstructure integrity, maintaining the intestinal steady-state, improving the activity of intestinal macrophages, inhibiting the level of inflammatory cytokines and preparing products for treating sepsis. The ADAR1 over-expressed virus vector disclosed by the invention provides novel potential therapeutic targets for treatment of sepsis.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Adenosine deaminase detection kit
InactiveCN105586387AGood colorRapid responseMicrobiological testing/measurementBiological material analysisPeroxidaseBovine serum albumin
The invention relates to an adenosine deaminase detection kit. A vessel is contained in the detection kit and filled with liquid for detection, the liquid for detection is prepared from 50-100 mmol / L of glycine buffer solution, 50-80 mmol / L of sodium benzoate, 12 / 18 u / L of purine nucleoside phosphorylase, 30-40 u / L of xanthine oxidase, 200-300 u / L of peroxidase, 30-100 g / L of glyceraldehyde, 0.5 g / L of sodium azide, 10-200 mmol / L of disodium hydrogen phosphate, 100-300 mmol / L of KCl, 40-100 mmol / L of mannitol, 0.1-0.3 ml / L of preservative, 100-150 mmol / L of adenosine, 2-4 mmol / L of 4-aminoantipyrine, 10-15 mmol / L of color developing agent, 20-40 mmol / L of adenine nucleoside and 3-5 g / L of bovine serum albumin. The detection kit has the advantages of being high in determination precision, high in antijamming capacity, suitable for automated rapid determination and capable of creating favorable conditions for routine development of ADA detection application in clinic.
Owner:VISION BIOLOGICAL TECH HEFEI CO LTD
Enzyme for synthesizing and metabolizing inosine monophosphate of Cordyceps sinensis(Berk.)Sacc. Hirsutella sinensis and application thereof
ActiveCN102690801AIncrease productionEnhance expressive abilityFungiBacteriaBiotechnologyBiosynthetic genes
The invention relates to an adenosine monophosphate (AMP) adenosine deaminase for synthesizing and metabolizing an inosine monophosphate from adenine nucleotide of Cordyceps sinensis(Berk.)Sacc. Hirsutella sinensis from 'Bailing' production strain, a gene coding the enzyme and application thereof. The AMP adenosine deaminase comprises the proteins shown in SEQ ID No. 1 and SEQ ID No. 2, and the coding gene of the AMP adenosine deaminase corresponds to the nucleotide sequence shown in SEQ ID No. 3 and SEQ ID No.4. According to the invention, the metabolizing way for synthesizing the inosine monophosphate from the adenine nucleotide is researched in detail from the principle, and the cloned DNA containing the nucleotide sequence provided by the invention can be transferred to an engineering strain by transduction, transformation, and combined transfer; the expression of the gene is biologically synthesized by regulating the inosine monophosphate, the host inosine monophosphate is endowed with high expressivity, an effective way is provided for increasing the yield of the inosine monophosphate, and the invention has a great application prospect.
Owner:ZHEJIANG UNIV OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com