ADAR1 (Adenosine Deaminases Acting on RNA1) over-expressed virus vector as well as construction method and application thereof
A virus vector and construction method technology, applied in the field of genetic engineering, can solve the problems of undisclosed ADAR1 application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The construction of embodiment 1 mouse ADAR1 expression vector
[0052] Due to the possible "off-target" phenomenon in RNAi experiments, we synonymously mutated the wild-type ADAR1 gene according to the RNAi target sequence, specifically cloning the sequence of mouse ADAR1 overexpressing adenovirus (Ad+O / E-ADAR1) : NM_001146296.1, ADAR1 low-expression adenovirus sequence (Ad-shADAR1): CCATGAACCTCGATTTAAA and negative control sequence (Ad-NC): TTCTCCGAACGTGTCACGT, carry out synonymous mutation on ADAR1 overexpression adenovirus sequence, and ADAR1 overexpression adenovirus sequence The sequence of the virus, the sequence of the ADAR1 low-expression adenovirus and the negative control sequence were respectively constructed with the lentiviral PCDH-CMV-MCS-EF1-copGFP plasmid as the backbone to construct the ADAR1 lentiviral expression vector.
Embodiment 2
[0053] Embodiment 2 establishes cecal ligation and puncture (CLP) sepsis mouse model
[0054] 1. Experimental animals
[0055] Select 6-week-old male C57BL / 6 mice, weighing 20-25g, and culture them under standard laboratory conditions.
[0056] 2. The experimental steps are as follows:
[0057] (1) On the morning of the operation day, the mice began to fast, and were anesthetized by intraperitoneal injection of 4% chloric acid hydrate. They were placed on the experimental operating table in a supine position, and after the abdominal skin was sterilized with 75% alcohol, a wound about 1 cm long was cut along the middle of the abdomen, and opened. The abdominal cavity finds the cecum and squeezes out the gas distal to the cecum.
[0058] (2) According to the design requirements of each group, a 3-0 line was used to ligate at 1 cm from the blind end or at the base of the cecum to avoid intestinal ischemia and necrosis caused by ligation of mesenteric vessels, and a 12-gauge nee...
Embodiment 3
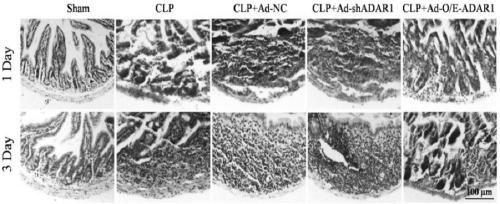
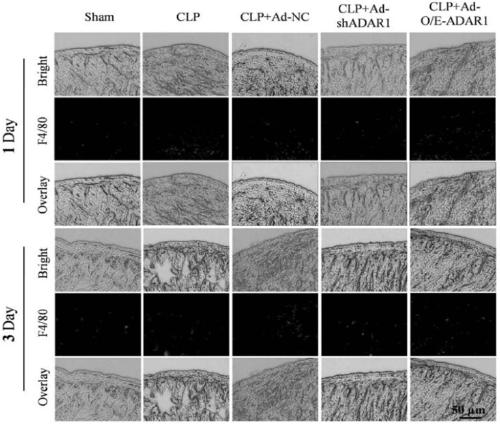
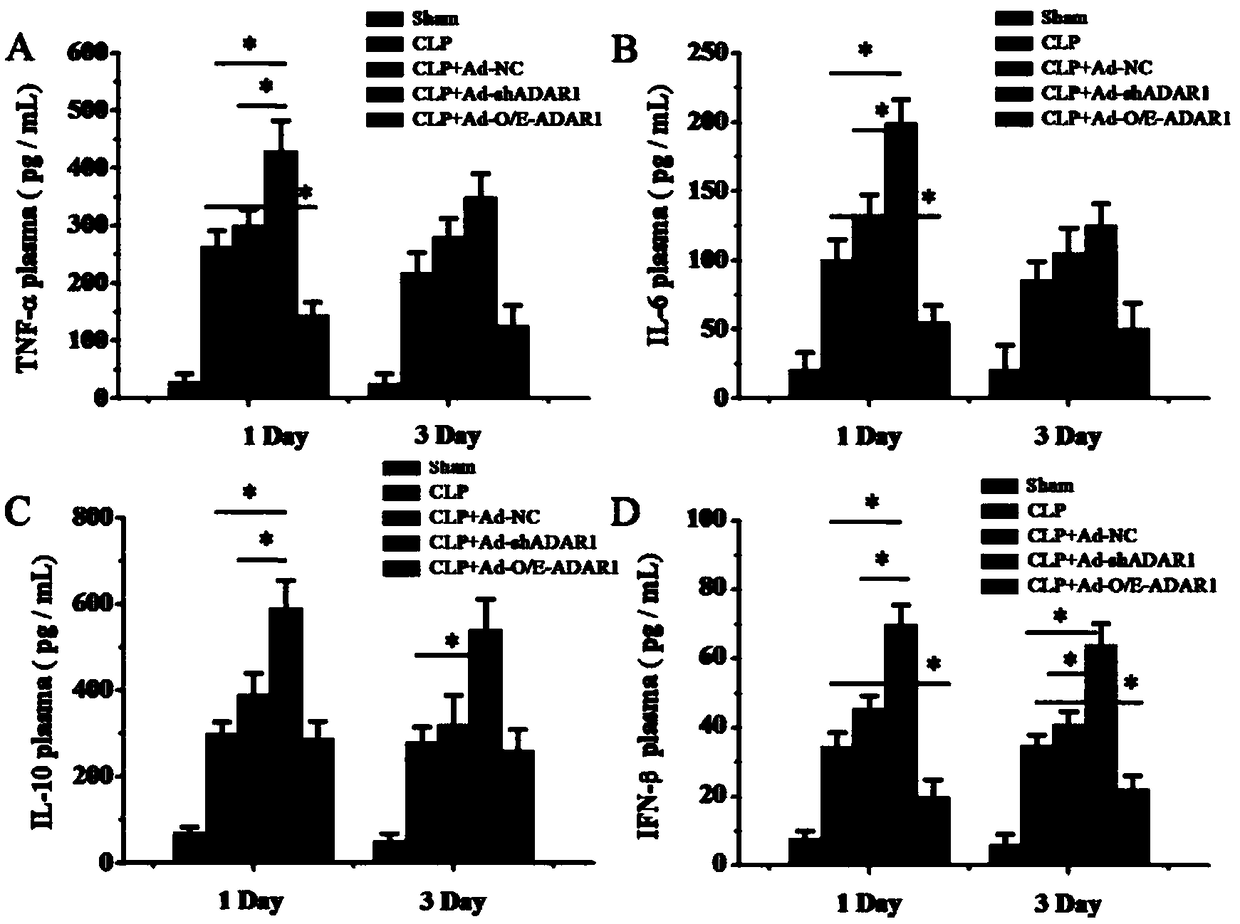
[0061] Example 3 Protective effect of ADAR1 overexpression virus vector on septic mice
[0062] 1. Experimental animal: the CLP sepsis mouse model prepared in Example 2.
[0063] 2. Experimental scheme: Divide the mouse models into five groups, put them in the fixer, expose the tail, and inject 100 microliters of ADAR1 overexpression adenovirus vector (containing 1×10 8 PFU ADAR1 overexpression virus), the same dose of low expression virus vector, the plasmid group without ADAR nucleic acid sequence, the same dose of PBS (sham model group, model group)---CLP+Ad-NC group. Mice were anesthetized on the first and third day after injection, and small intestinal samples were collected for histological analysis.
[0064] 3. Experimental results
[0065] CLP induced sepsis in mice, respectively overexpressed / disturbed ADAR1 in the small intestine, and H&E staining was used to evaluate the histomorphology of the small intestine of septic mice. figure 1 The results showed that after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com