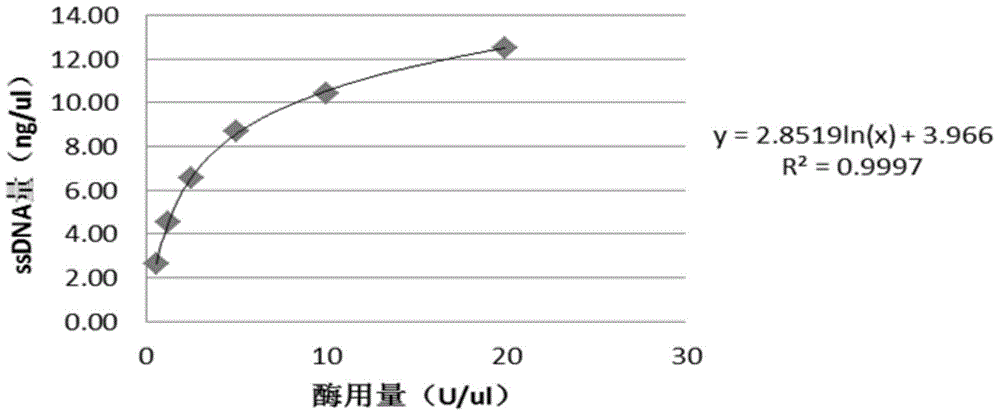
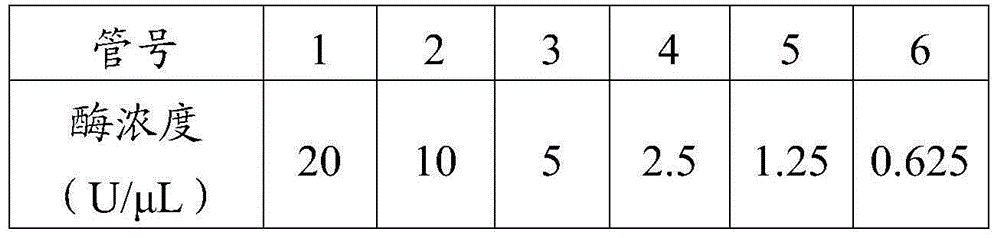
Patents
Literature
194results about How to "No radioactive contamination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Artificial stone panel and manufacturing method thereof
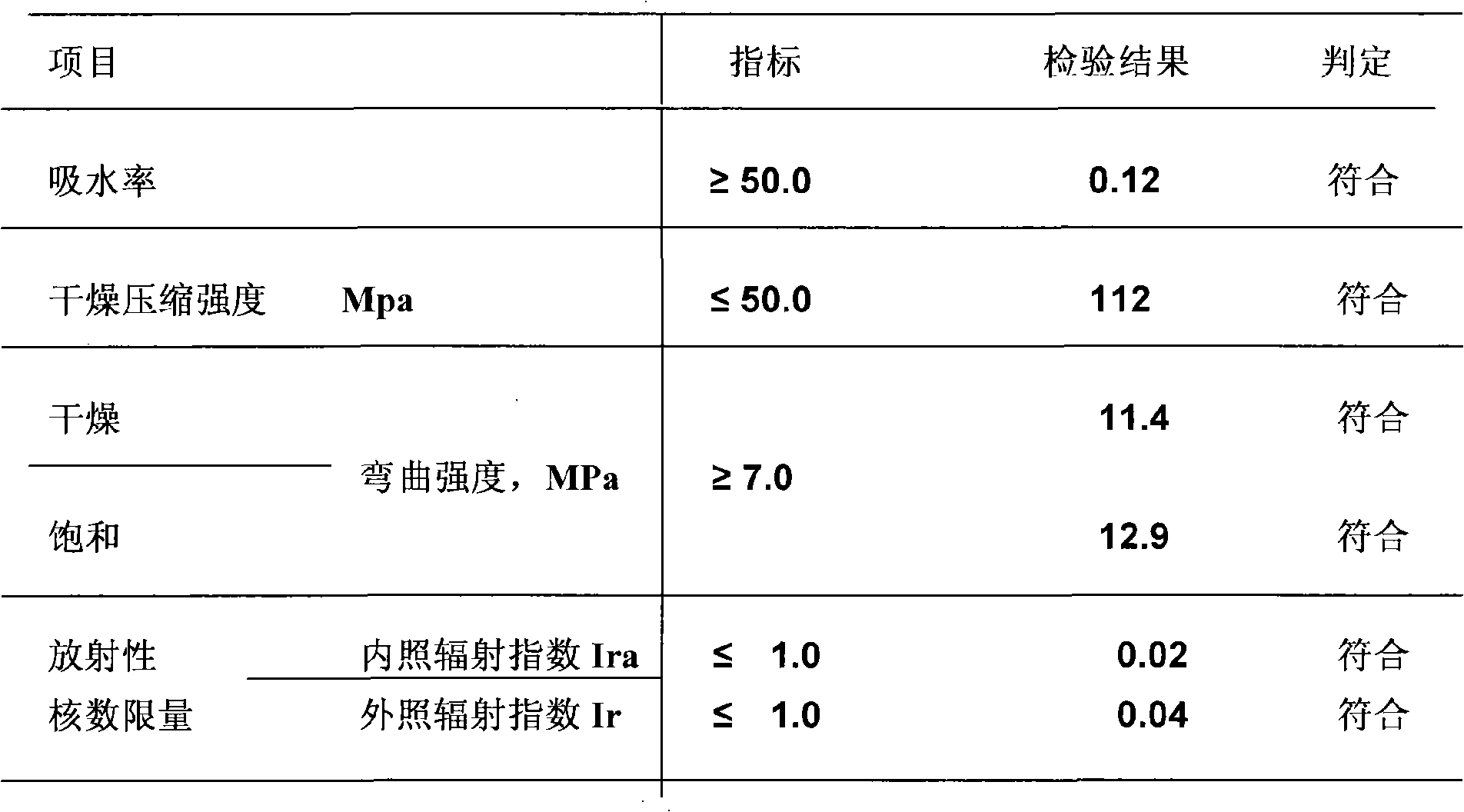
InactiveCN101328039AGood wear resistanceNoble and elegant decorative effectCalcium carbonateArtificial stone
The invention discloses a artificial stone plate prepared by adding cobalt naphthenate accelerator, methylethylketone, light stabilizer, paint and antioxidant into unsaturated polyester resin. The preparation method includes the following steps: firstly modifying the aluminium hydroxide and / or calcium carbonate to get surface modified aluminium hydroxide and / or calcium carbonate or pure aluminium hydroxide powder, then adding cobalt naphthenate accelerator, methylethylketone, light stabilizer, paint and antioxidant into unsaturated polyester resin, uniformly mixing and stirring the mixture in a high speed stirring reactor, adding surface modified aluminium hydroxide and / or calcium carbonate powder or pure aluminium hydroxide powder, stirring and casting the mixture into a mould after vacuum defoamtion, heating up to 60-80 DEG C and curing to get the artificial stone and marble panel.
Owner:覃庆峰
Application of cleavable polyethylene glycol (PEG) lipid derivative to preparation
InactiveCN102068701AEasy to prepareNo radioactive contaminationPowder deliveryPharmaceutical non-active ingredientsLiposome VesicleMethyl carbonate
The invention belongs to the technical field of medicaments, and provides application of a cleavable polyethylene glycol (PEG) lipid derivative to preparation of a PEGylated preparation for relieving or avoiding accelerated blood clearance. In the application, liquid microparticle preparations such as liposome, vesicles, emulsions, microemulsion, micelles, nanoparticles and the like are modified by the cleavable PEG lipid derivative such as PEG-cholesteryl hemisuccinate, PEG-cholesteryl methyl carbonate, PEG-alpha tocopheryl hemisuccinate and the like; and the measurement of variation of preparation elimination in tissues such as animal blood plasma, liver, spleen and the like after a cleavable PEG lipid derivative-modified medicinal preparation is repeatedly injected proves that repeated injection of cleavable PEG lipid derivative-modified microparticle preparations only causes light accelerated blood clearance or avoids the accelerated blood clearance, namely the accelerated blood clearance can be relieved or avoided. The invention discloses new application of the cleavable PEG lipid derivative.
Owner:SHENYANG PHARMA UNIVERSITY
Page quantitative determination device and methods based on terahertz time-domain spectroscopic technology
InactiveCN101178355ASuitable for quantitative detectionImprove signal-to-noise ratioColor/spectral properties measurementsSpecial data processing applicationsData acquisitionPolarizer
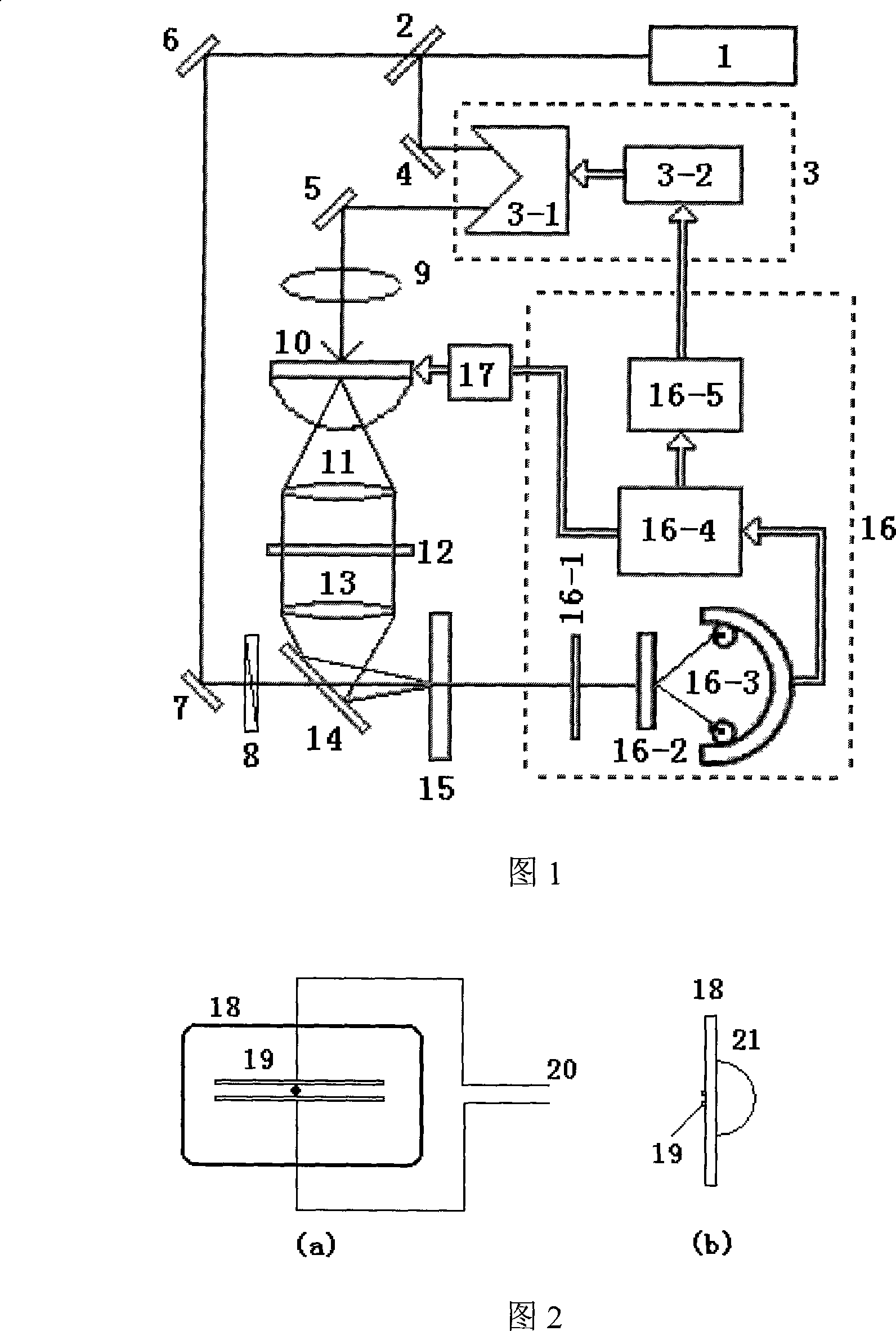
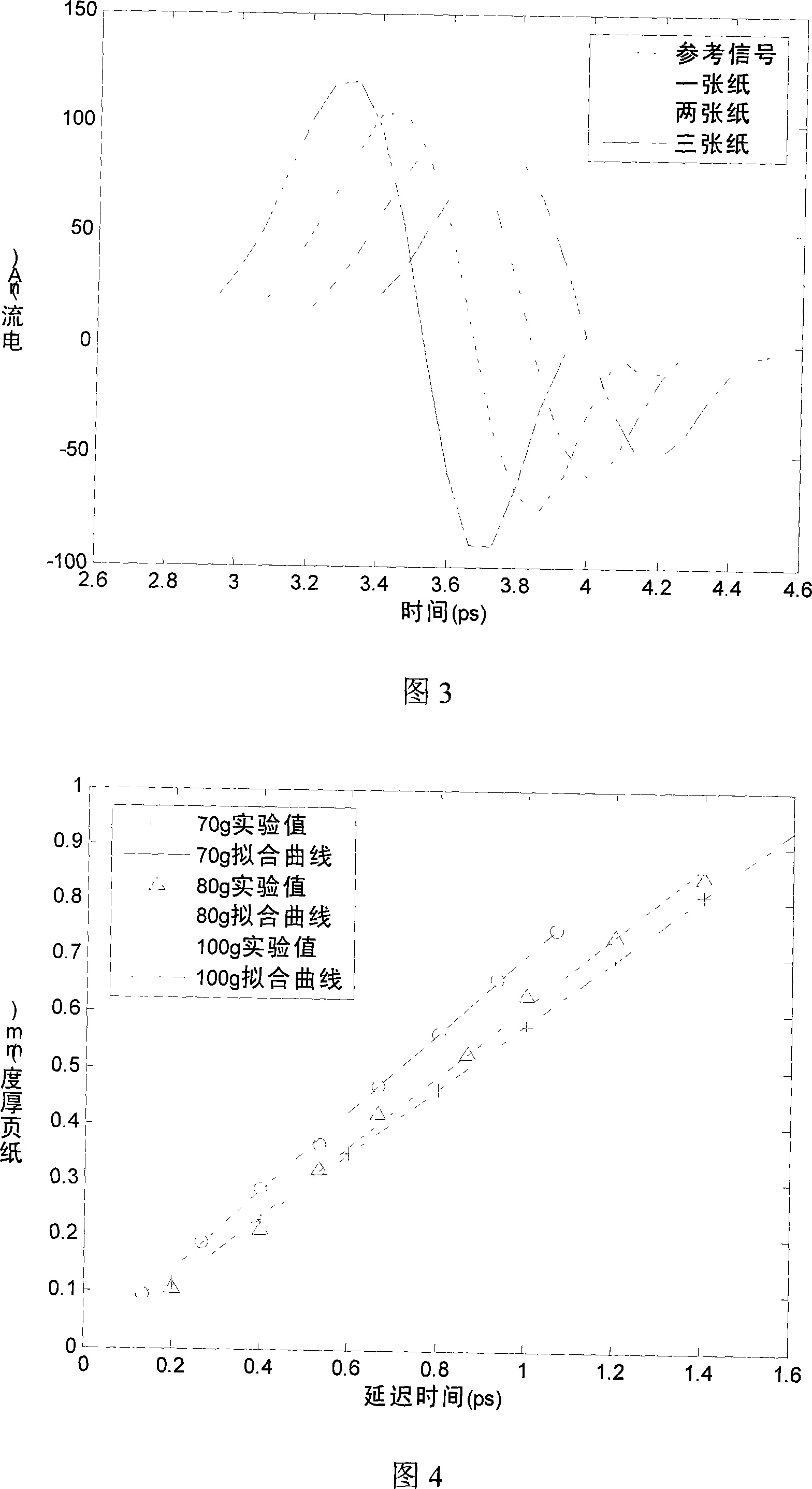
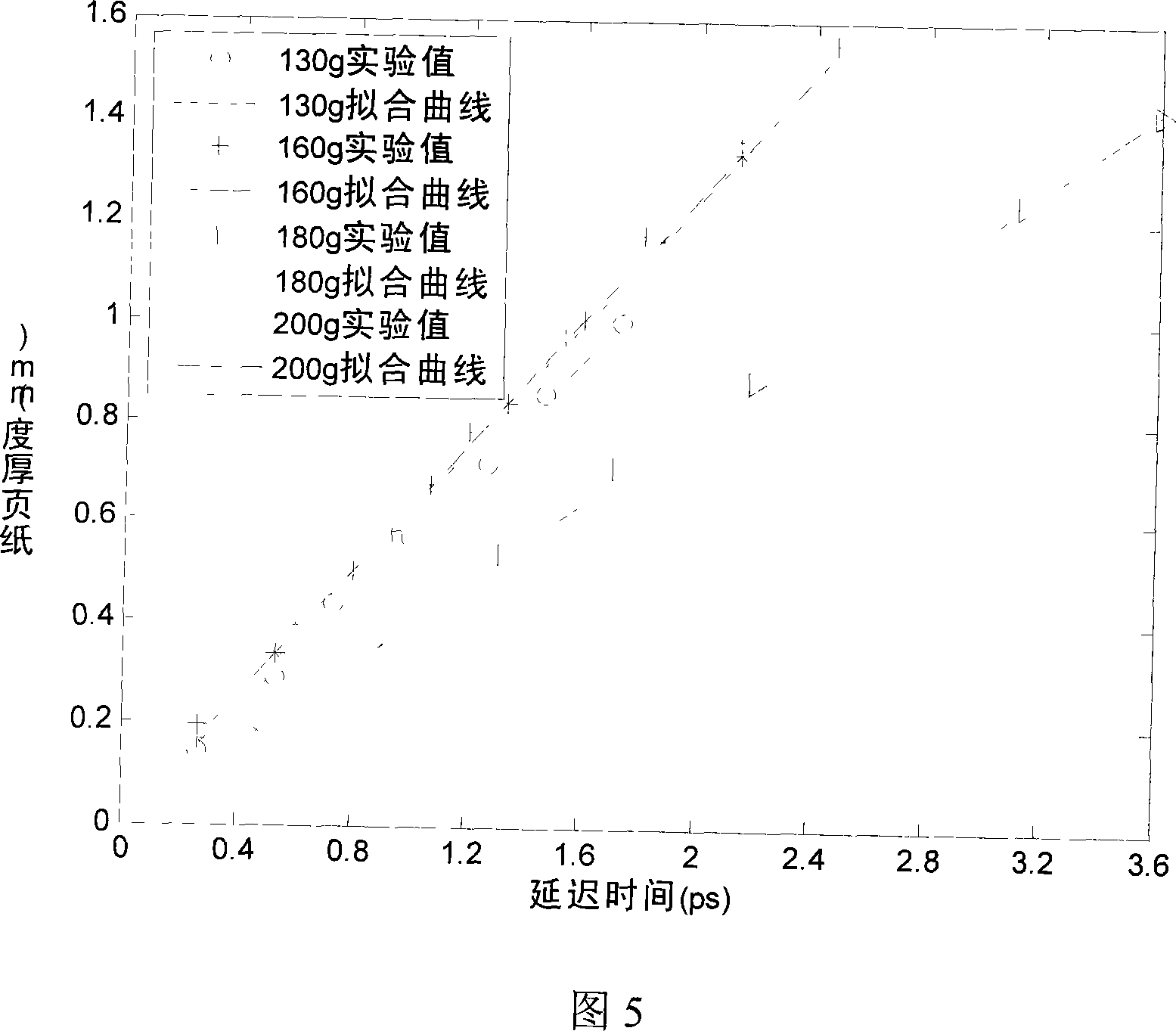
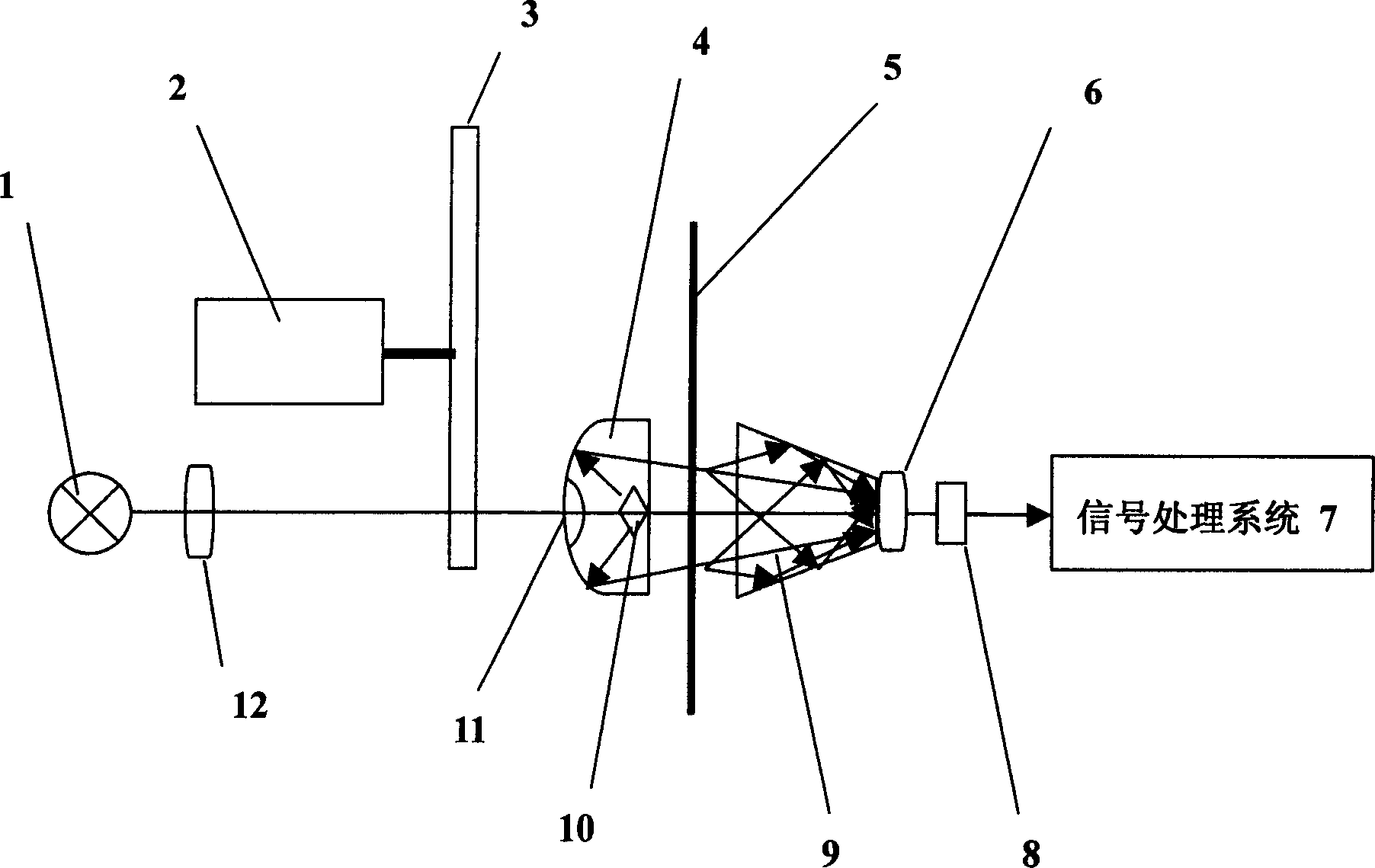
The invention discloses a paper sheet quantitative detection device which is based on a terahertz time-domain spectroscopy technology and a method thereof. The detection device includes: a femtosecond laser, a beam splitter, an optical delay device, a reflective mirror, a focusing lens, a photoconductive antenna type terahertz transmitter, a polyethylene lens, a polarizer, a zinc telluride electro-optical crystal, a phase-locking amplifier, a high-power amplifier and a data collection and processing system. The invention makes use of the polyethylene lens for aligning the terahertz waves to the parallel light beams, and the polyethylene lens is used for focusing the terahertz waves on a terahertz detector after penetrating the paper sheet. The paper sheet quantitative detection method which is disclosed by the invention firstly establishes a paper sheet quantitative detection mathematical model, then respectively measures the time-domain signals of the terahertz waves penetrating the air and penetrating the paper sheet, extracts the delay time and applies the established mathematical model to calculate the paper sheet quantitative value. The invention has high precision of the measurement, strong anti-interference capability, small influence of multiple reflections and safe usage, so the invention can be used in the precise paper sheet quantitative detection of the field of paper making.
Owner:ZHEJIANG UNIV
Method for producing and using water-preserving agent used in desert
InactiveCN1465650APrevent leakageImprove survival rateOrganic fertilisersSoil conditioning compositionsCALCIUM CARBONATE/MAGNESIUM CARBONATEPhosphate
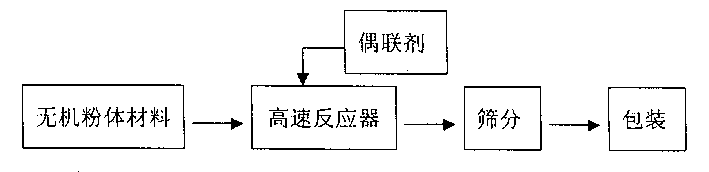
The water-preserving agent for desert zone is made of inorganic powder material and coupling agent according to the weight ratio of 100:0.5-2.5, the described inorganic powder body material is any one of calcium carbonate, magnesium carbonate, talcum powder, pottery clay, wollastonite, coal gangue, flyash, diatomaceous earth or desulfurized gypsum or their any combination, and the described coupling agent is any one of coupling agent of organic titanate, RCA modifying agent, aluminate coupling agent, phosphate coupling agent or silane coupling agent.
Owner:曾敦华
Chemical luminescence immune assay determination reagent kit for detecting human growth hormone
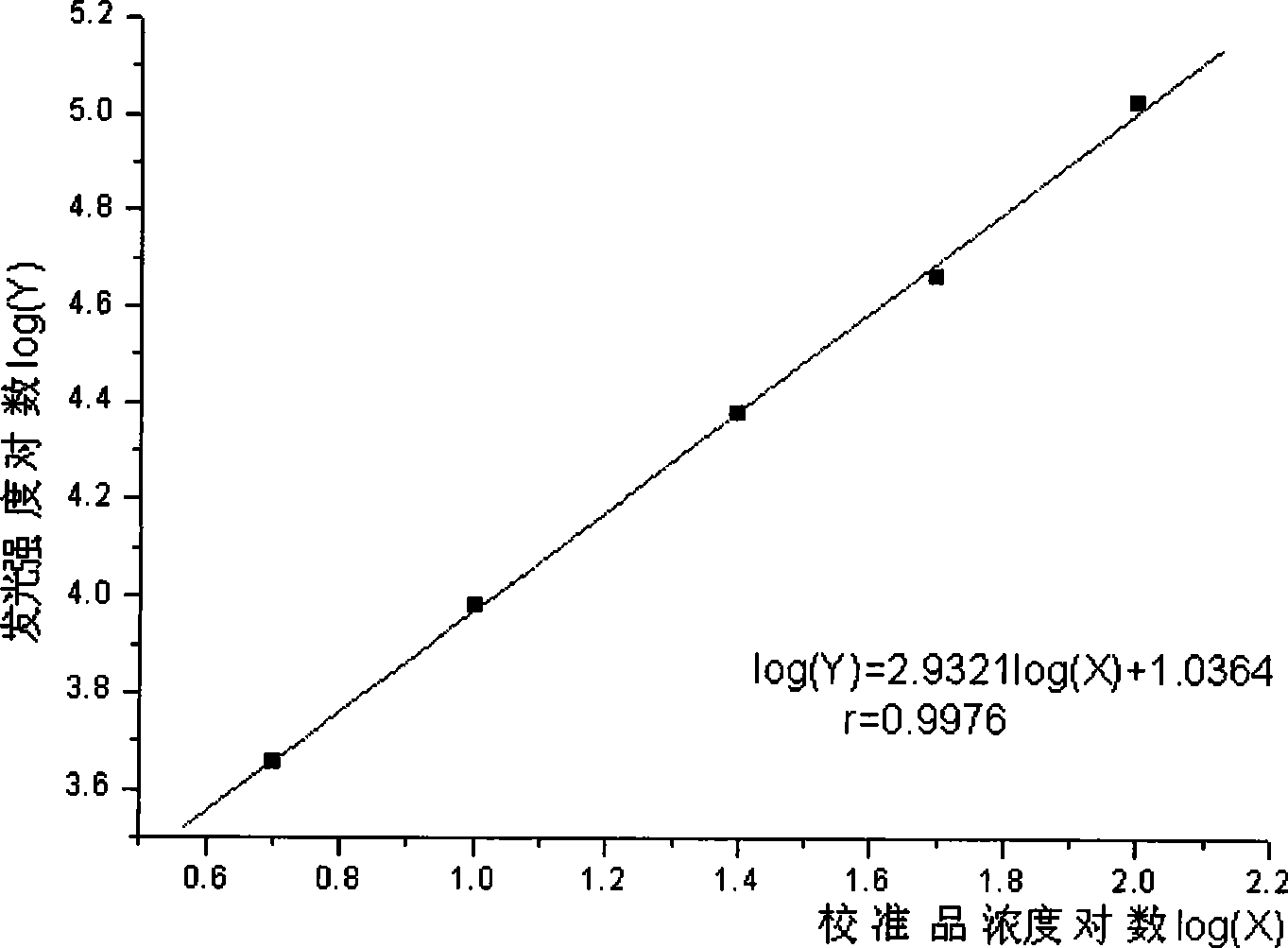
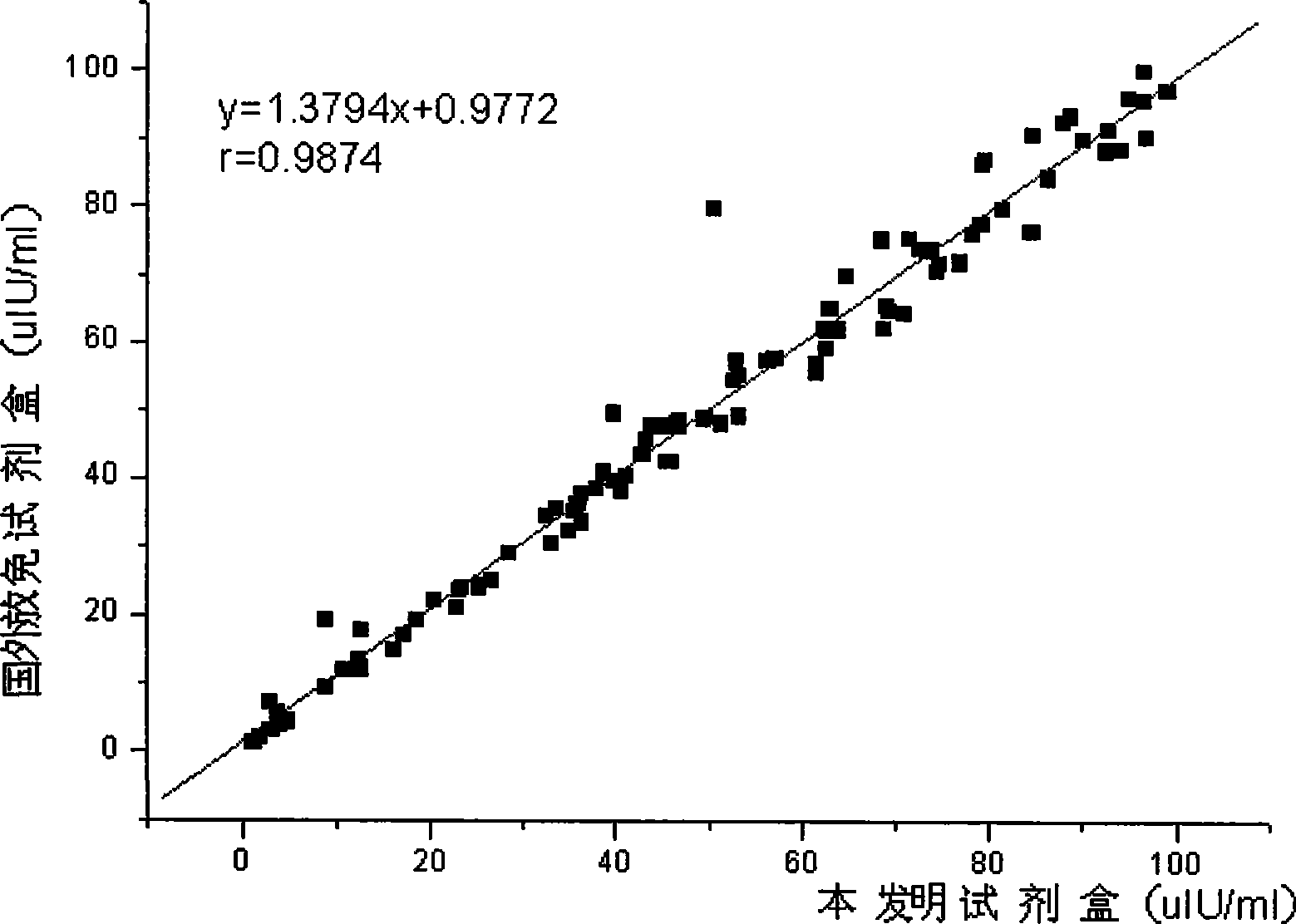
InactiveCN101368973AImprove performanceEasy to operateChemiluminescene/bioluminescenceBiological testingLuminescencePollution
The invention relates to the immunoassay field, in particular discloses a chemiluminescence immunoassay test kit and a preparing method thereof for detecting human growth hormone. The test kit uses principle of enzyme-catalyzed chemiluminescence, and adopts the micro hole plate as the solid-phase carrier so as to achieve batch detection. The monoclonal antibody can be labeled by alkaline phosphatase and horseradish peroxidase. Without radioactive pollution, the test kit has stable performance, simple operation, accurate and sensitive result and swift reaction speed.
Owner:北京科美东雅生物技术有限公司
Near infrared quantitative water content measurement method for paper
ActiveCN1800825AEnables real-time process detectionNo radioactive contaminationColor/spectral properties measurementsAudio power amplifierEngineering
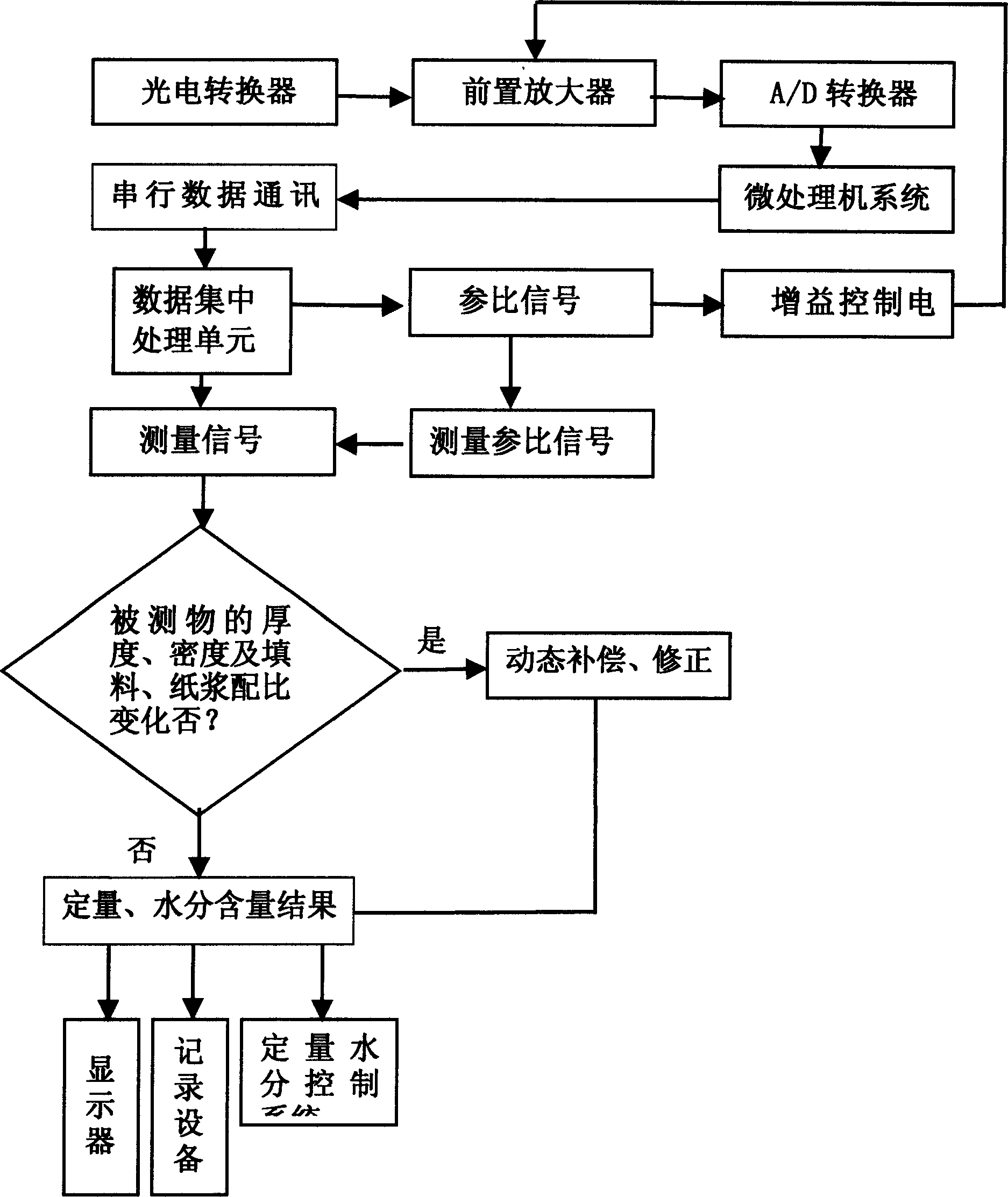
The quantitative moisture measurement method for near infrared paper comprises: modulating the infrared light into near infrared lights with different wavelength and arranged as chronological order to reflect with a reflector to the emission integral semisphere to reflect and cross the paper to focus on a optical-electrical converter, sending the converted electric signal to microprocessor for pretreatment; then, transmitting into a data centralized processing unit to recognize and divide into reference signal with one path to control front amplifier and another path as the reference signal and measurement signal; calculating in the processing unit to obtain quantitative moisture content. This invention realizes online measurement with high accuracy and low cost.
Owner:西安力源光电科技有限责任公司
Floor, manufacture method and application thereof
ActiveCN102757590ANo radioactive contaminationMeet the testing standardsFlooringRed mudPolyvinyl chloride
The invention provides a floor with industrial residues as main materials. The materials for manufacturing the floor include red mud, functional additives and enhancing carriers. The component includes, by weight, 55 to 70 parts of red mud, 30 to 45 parts of plastic, 0.05 to 4 parts of functional additives A, 3 to 5 parts of functional additives B and 3 to 5 parts of enhancing carriers, wherein the plastic is selected from polyethylene, polrvinyl chloride or polypropylene; the functional additives A are selected from impact modifier chlorinated polyethylene (CPE), plasticizer dioctyl adipate (DOA), and nucleating agent beta crystal form nucleating agent tetramethylbenzidine (TMB)-5; the functional additives B are selected from a processing modifier ethylene propylene diene monomer (EPDM), and an impact modifier methyl methacrylate-butadiem-styrene (MBS) copolymer resin; and the enhancing carriers are E glass fibers. The floor has good corrosion resistance, firmness and light quality, water proofing, fire proofing, electricity proofing properties, sound insulation and heat insulation performances, no radioactive contamination or harmful gas, and good market prospects and use values.
Owner:湖北声荣再生资源利用有限公司
Building insulated wall body material and preparation method thereof
The invention relates to a building insulated wall body material and a preparation method of the building insulated wall body material. The preparation method comprises the following steps of: (1) mixing ardealite, red mud, fly ash, cement, yellow sand and lime according to the proportion, and adding a foamer so as to obtain a material which is uniformly mixed; (2) adding water in the material after mixing, performing ball milling, and preparing a slurry;(3) pouring the slurry after ball milling in a mould so as to carry out injection molding; and (4) placing a green body in a maintenance box, maintaining under high temperature and high pressure, and then maintaining under a natural state. Compared with the prior art, the building insulated wall body material and the preparation method have the advantages that the industrial residue, the ardealite and the red mud are sufficiently utilized, the pollution of the ardealite and the red mud to the environment can be reduced, and thus the purpose of recycling resources is achieved. The manufacturing of the building heat insulating material has the advantages of short period, low cost, no radioactive contamination, low density and high strength.
Owner:武汉中理环保科技有限公司
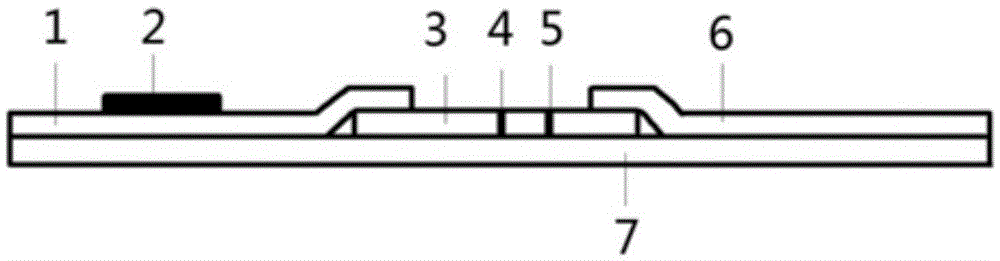
An NGAL time-resolved fluoroimmunoassay nanometer immunochromatographic quantitative detection test paper strip and a preparing method thereof
InactiveCN105527439AReduce penetration rateGuaranteed accuracyMaterial analysisFluorescenceRadioactive contamination
The invention discloses an NGAL time-resolved fluoroimmunoassay nanometer immunochromatographic quantitative detection test paper strip and a preparing method thereof. The test paper strip comprises a backing substrate plate. The backing substrate plate is provided with a nitrocellulose membrane, a sample combining pad and a water absorbing membrane. The sample combining pad and the water absorbing membrane are respectively laminated on two ends of the nitrocellulose membrane. The sample combining pad is covered with an NGAL monoclonal antibody labeled with a time-resolved fluorescent substance. The nitrocellulose membrane is provided with a detection line and a quality control line. The detection line is covered with an NGAL monoclonal antibody of another epitope. The quality control line is covered with goat anti-rat IgG. The test paper strip and the method are characterized by high sensitivity, high specificity, good stability, no radioactive contamination, and the like and can be widely applied to NGAL clinical immunoassay and scientific research.
Owner:XIAMEN YIKELISI MEDICAL TECH CO LTD
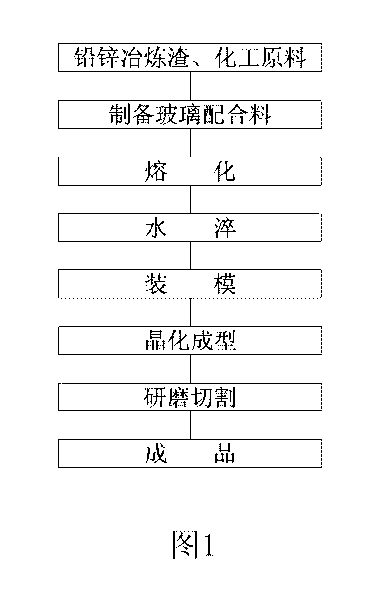
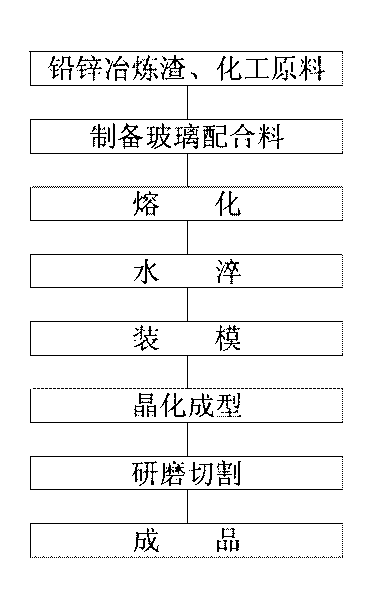
Green glass-ceramic plate made from lead-zinc smelting slag and manufacturing method thereof
The invention relates to a green glass-ceramic plate made from lead-zinc smelting slag and a manufacturing method thereof. The glass raw material is prepared from the following components in parts by weight: 40 to 60 parts of lead-zinc smelting slag, 26 to 38 parts of quartz, 15 to 24 parts of lithium feldspar, 2 to 4 parts of soda ash, 2 to 3 parts of zinc oxide, 2 to 5 parts of barium carbonate, 1 to 3 parts of borax, 2 to 2.5 parts of chromium oxide, 0 to 0.2 part of copper oxide and 0.04 to 0.2 part of carbon powder. The invention is not only in line with the common glass-ceramic produced by completely adopting industrial materials on the aspect of the performance, but also can realize waste utilization, reduce the environmental pollution, the product cost and the melting temperature of glass, improve the melting speed and the melting furnace efficiency of the glass, decrease the energy consumption and can be widely applied to building decoration materials, especially applied in various building decoration engineering, such as inside and outer walls, floors, pillars, table boards of the building, and the like.
Owner:FENGXIAN SANLIAN BUILDING MATERIALS
Insulin and C peptide double-tagging determination kit
InactiveCN102419373ASolve the problem of unstable storage at 28°CSolve the unstable placementBiological testingFluorescence/phosphorescenceC-peptideEuropium
The invention relates to an insulin and C peptide double-tagging kit, especially relates to a kit for determining insulin and C peptide by a double-tagging time resolution immunofluorescence and by using dried blood spots specimen on filter paper as a sample, and provides a preparation method of europium marked anti C peptide antibody and samarium marked insulin antibody. The kit can carry out simultaneous quantitative detections on two landmarks of insulin and C peptide through one detection, and the sample of dried blood spots specimen on filter paper solves a problem of unstable insulin and C peptide in a serum sample. The kit of the invention can be widely applied to various researching field of aided diagnosis and patient's condition detection, clinical drug guidance and prognosis of diabetes.
Owner:GUANGZHOU DARUI BIOTECH
Dissociate human chorionic gonadotrophin beta-subunit magnetic particle chemiluminescence quantitative assay kit and its preparation method
InactiveCN103076458AGuaranteed SensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingImmune profilingChromogenic Substrates
The invention relates to the medical field of immunoassay, and concretely provides a dissociate human chorionic gonadotrophin beta-subunit magnetic particle chemiluminescence quantitative assay kit having the advantages of simplicity, rapidness, high sensitivity, wide linear range and good stability, and its preparation method. The method combines a magnetic particle immune separating technology to apply an enzymatic chemiluminescence substrate on the basis of enzyme-linked immunosorbent assay, and allows an optical signal generated by the detection of the luminescence substrate to substitute a chromogenic substrate in enzyme immune assay, so the method has the advantages of substantially improved sensitivity, simple operation and wide practicality, can be applied to open semi-automatic chemiluminescence detectors, can also be applied to full-automatic measure systems, and can realize batch and fast detection, low use cost, and easy popularization and application.
Owner:BEIJING LEADMAN BIOCHEM
Calcium sulfate-whisker compounded quartz stone board product and preparation method thereof
InactiveCN103408251AImprove performanceNo radioactive contaminationAnhydrous Calcium SulfateGlass particle
The invention discloses a calcium sulfate-whisker compounded quartz stone board product, which comprises the following materials in parts by weight: 58-75% of particle raw material, 10%-25% of quartz powder, 0.01%-10% of pigment, 0.1%-1% of silane coupling agent, 8%-12% of unsaturated polyester resin, 5%-10% of anhydrous calcium sulfate, and 0.8-2% of curing agent, wherein the particle raw material is one or two selected from quartz particles and glass particles. The product is an environment-friendly novel decoration material which has high property and no radioactive contamination, can be recycled, and has the characteristics of gay colors, high finish degree, uniform color, pressure-resistance and abrasion-resistance, good toughness, compact structure, low water absorption, corrosion-resistance, high strength, impact-resistance, innocuousness and zero radiation, and the like.
Owner:GUANGDONG BANNER NEW MATERIAL TECH
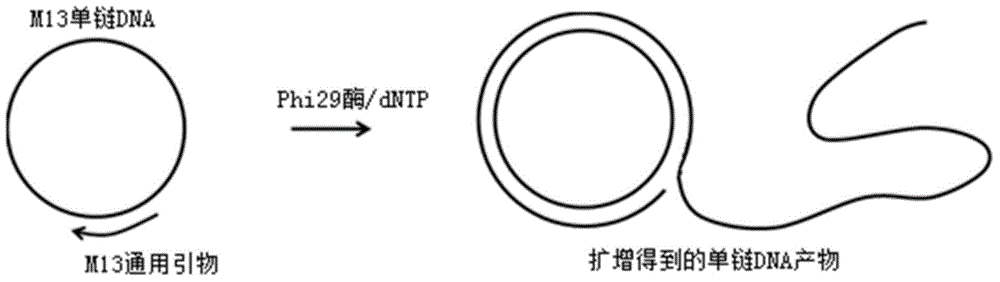
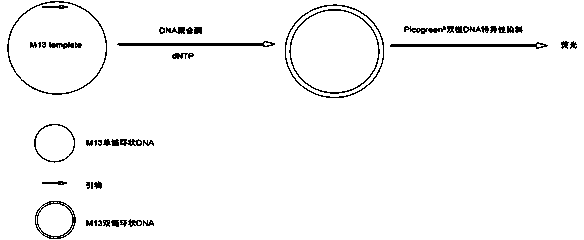
Method for measuring activity of Phi29 DNA polymerase and rolling circle sequencing method
InactiveCN106319032AEnhance aggregation abilityHigh activityMicrobiological testing/measurementHigh fluxFluorescence
The invention discloses a method for measuring the activity of Phi29 DNA polymerase and a rolling circle sequencing method. The method for measuring the activity of Phi29 DNA polymerase comprises the following steps: taking a circular single-stranded DNA as a template; in the presence of an amplification primer, using Phi29 DNA polymerase to trigger rolling circle replication to generate a large amount of continuous single-stranded DNA copies; then doping fluorescent molecules into the amplification products, detecting the fluorescent signals of the amplification products to quantify the produced single-stranded DNA copy number, and thus calculating the activity of Phi29 DNA polymerase. The provided method has the advantages of no radioactive contamination, simpleness, rapidness, and high sensitivity, and can be applied to high-flux and automatic polymerase screening.
Owner:MGI TECH CO LTD
Building thermal insulation wall body material and preparation method thereof
InactiveCN102531667AEfficient use ofReduce pollutionSolid waste managementCeramicwareFoaming agentThermal insulation
The invention relates to a method for preparing a building thermal insulation wall body material by utilizing industrial waste red mud, iron tailings, feldspars and yellow sand through a high temperature foaming method. The building thermal insulation wall body material mainly comprises the following materials by mass percent: the red mud accounts for 20 to 40 percent, the iron tailings account for 15 to 30 percent, the feldspars account for 20 to 30 percent, the yellow sand accounts for 12 to 20 percent, and inflating agents account for 2 to 8 percent. The method for preparing the building thermal insulation wall body material comprises the steps such as material mixing, blank body preparation, high-temperature foaming and the like. The method for preparing the building thermal insulation wall body material is of great significance in aspects of efficiently utilizing industrial waste residues, reducing the environmental pollution caused by the industrial waste residues and lowering the manufacturing cost; and meanwhile, the produced building thermal insulation wall body material is light and porous, achieves high intensity, and achieves functions such as better heat preservation, heat insulation, acoustic insulation and the like.
Owner:姜全辉
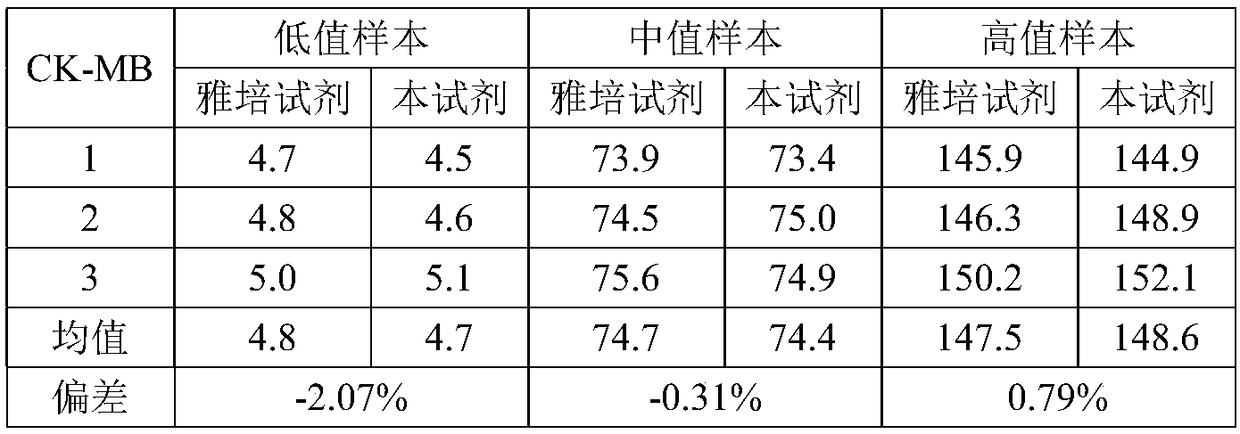
Magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB (creatine kinase-MB) kit
The invention discloses a magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB kit. The CK-MB kit comprises an immunomagnetic bead coating a CK-MB monoclonal antibody, a CK-MB standardized product solution, a europium-marked CK-MB monoclonal antibody solution, washing liquid and enhancement liquid. The immunomagnetic bead coating the CK-MB monoclonal antibody isa covalent conjugate of a superpara magnetic bead modified by a functional group and with the diameter being 1-3 microns and the CK-MB monoclonal antibody. The kit has the high sensibility, the sensibility of CK-MB is 1ng / mL, and a blood serum (plasma) does not need to be diluted; the determination time is short, and a report can be resulted within 30 minutes; the demanding amount of the sample isless, and only 50 microliters are needed for one-time sample loading; and the kit is equipped with a full-automatic time resolution immune analysis meter, operation is easy, no artificial error exists, and labor is saved. The kit reasonably utilizes the space of a reagent strip, the structure of the reagent strip is more compact, the reagent strip can be transported more easily, and used conveniently, the operation is simple, and the stability is good.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
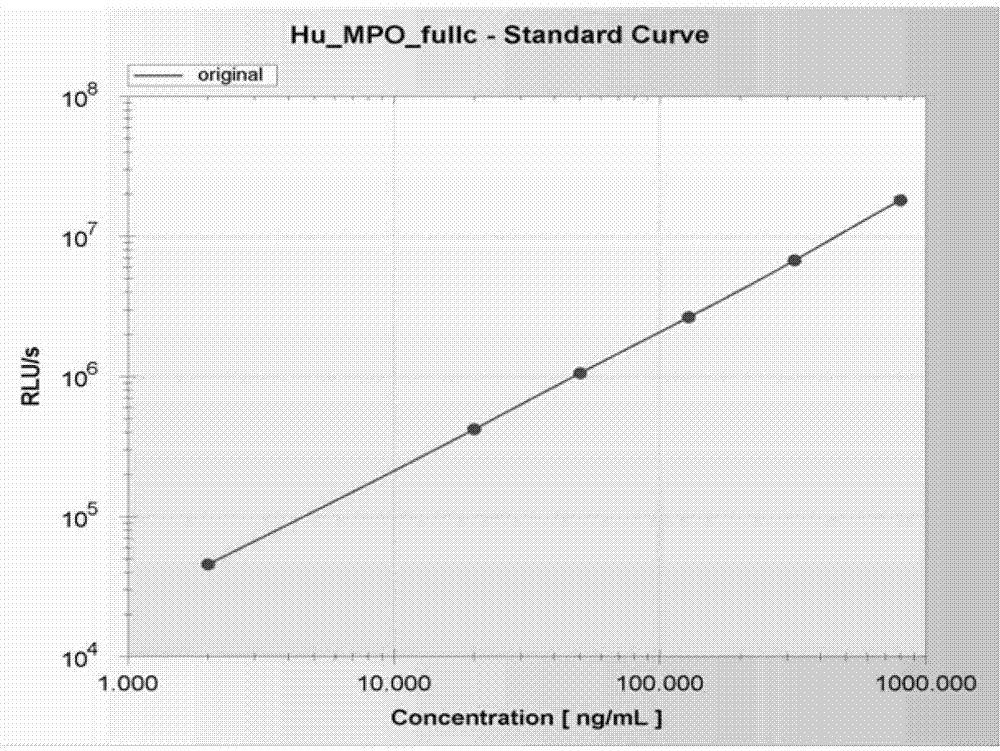
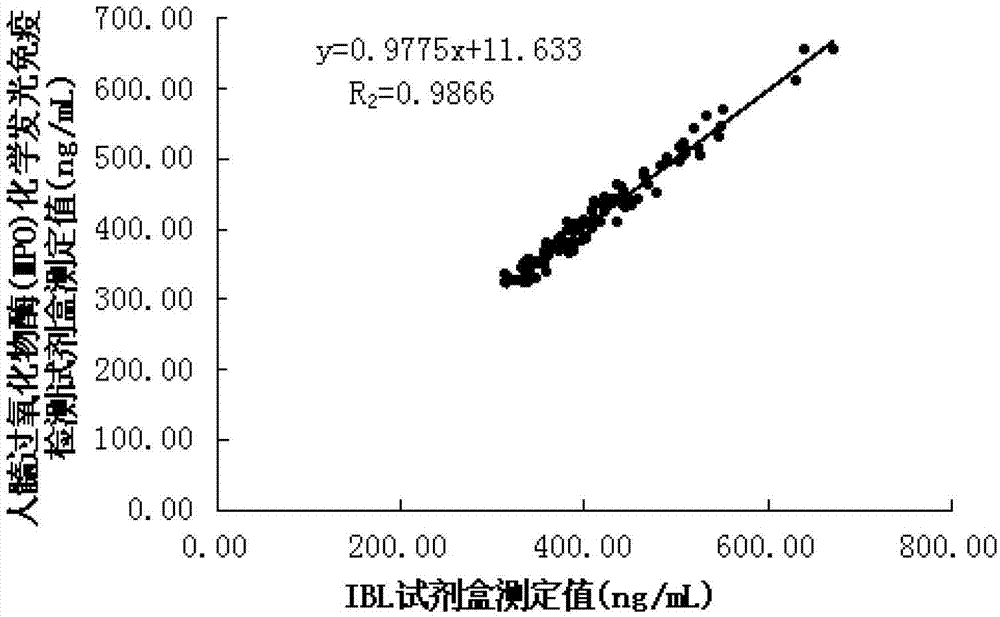
Human myeloperoxidase chemiluminescent immunodetection kit
InactiveCN103033624AHigh sensitivityImprove stabilityChemiluminescene/bioluminescenceChemistryACS - Acute coronary syndrome
The invention discloses a human myeloperoxidase (MPO) chemiluminescent immunodetection kit. The kit comprises the following components: 1) a human MPO standard product; 2) a vector coating a human MPO monoclonal antibody; 3) the enzyme-labeled human MPO monoclonal antibody; 4) a chemiluminescent substrate on which the enzyme acts; and 5) a concentrated cleaning solution. The kit can perform concentrated quantitative detection on the MPO molecules in a serum sample of a patient. The kit effectively utilizes a chemiluminescent technology principle and adopts the enzyme to catalyze the chemiluminescent substrate on the basis of enzyme-linked immunoassay so as to improve the detection sensitivity, has the advantages of high stability, high specificity, excellent accuracy, simplicity in operation and no radioactive pollution, and can provide specific, quick and reliable basis for clinical diagnosis of cardiovascular diseases such as atherosclerosis (AS), acute coronary syndrome (ACS) and coronary heart disease (CHD).
Owner:天津市协和医药科技集团有限公司
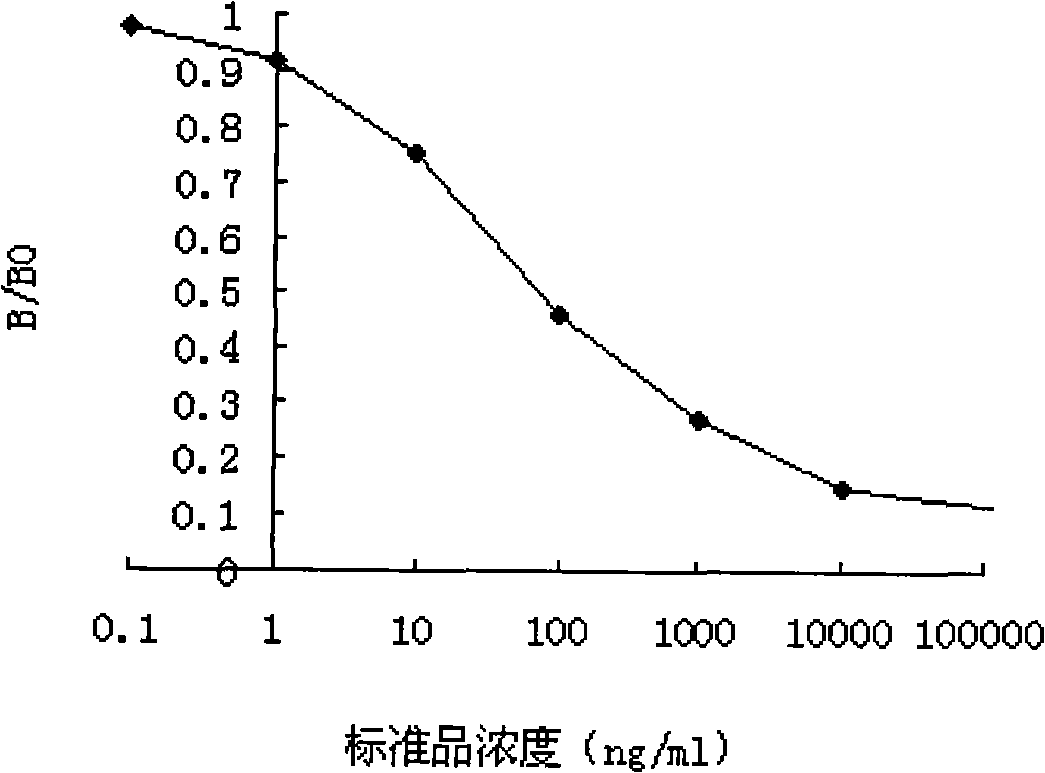
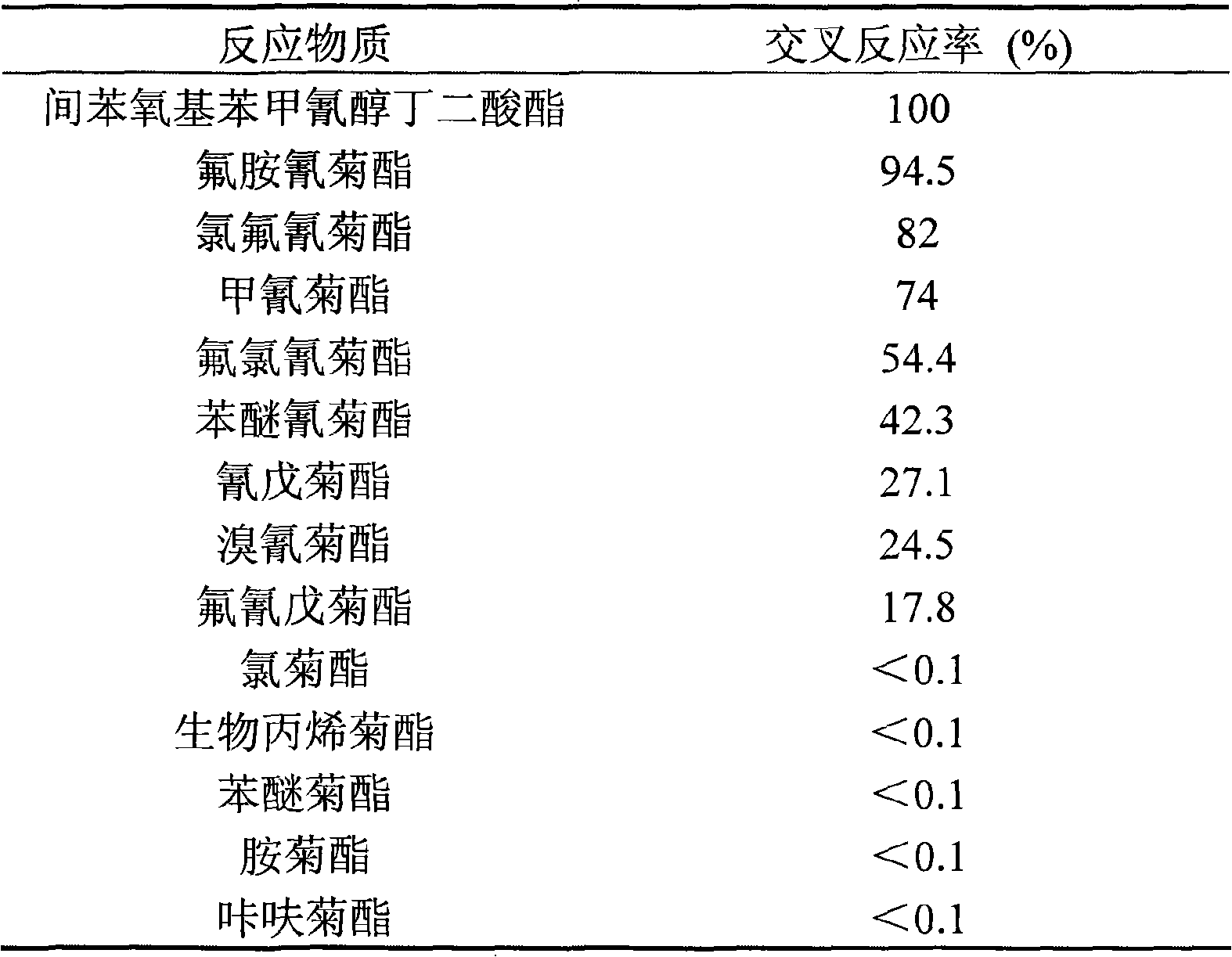
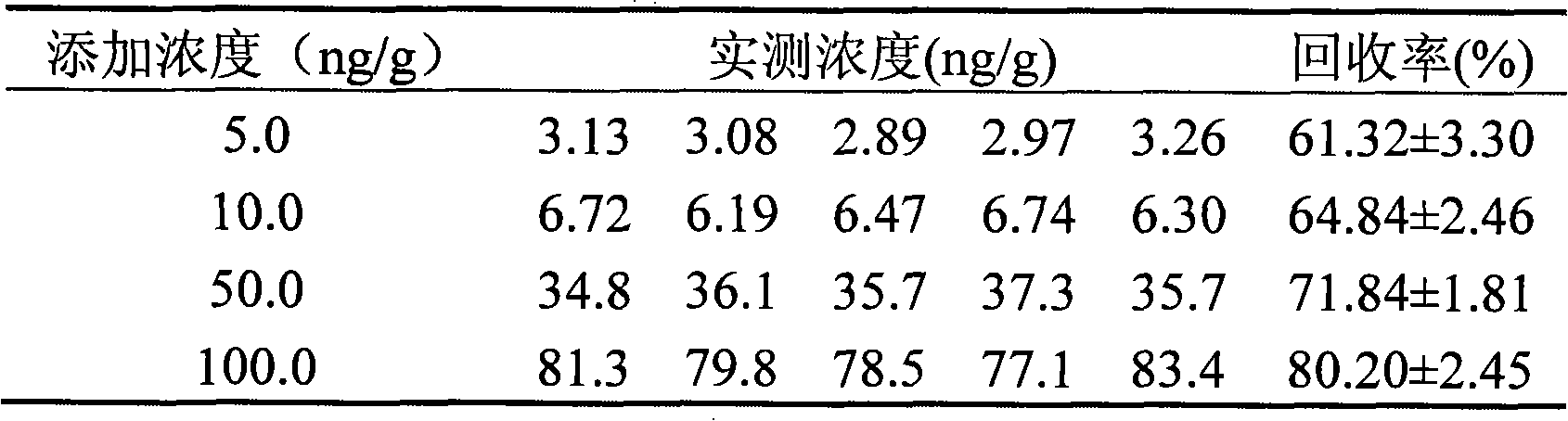
Enzyme linked immunological adsorption detection method for analyzing residuals of cyano pyrethroid pesticides
InactiveCN101334408ASensitive and accurate detectionThe pre-processing process is simpleMaterial analysis by observing effect on chemical indicatorTime-ConsumingPollution
The invention discloses an enzyme-linked immunosorbent assay method for multi-residue analysis of a cyano-containing pyrethroid pesticide, belonging to the technical field of immunoassay. The enzyme-linked immunosorbent assay method uses a coupled complex of hapten m-phenoxy benzal cyanohydrin succinate ester and ovalbumin as a coating antigen, samples of the cyano-containing pyrethroid and a polyclonal antibody of the m-phenoxy benzal cyanohydrin succinate ester are added, then the coating antigen and the cyano-containing pyrethroid to be detected are competed and reacted with the antibody, an enzyme-labeled secondary antibody is added to be combined with the fixed antibody, washing liquid is used for washing, developer is added, an enzyme-labeled instrument is used for detecting OD value, the OD value is compared with a standard curve of a standard product of the cyano-containing pyrethroid, and the concentration of the cyano-containing pyrethroid of the samples to be detected is calculated. The enzyme-linked immunosorbent assay method can accurately and sensitively detect the residues of the cyano-containing pyrethroid pesticide in the samples to be detected, the pre-treatment process of the samples is simple with less time-consuming, a large number of samples can be simultaneously detected, and the detection cost of the samples is far lower than the detection method of the traditional instrument; furthermore, the method of the invention has good stability and no radioactive pollution.
Owner:JIANGNAN UNIV
Double-tagging time resolution fluoroimmunoassay reagent kit based on PG magnetic particle
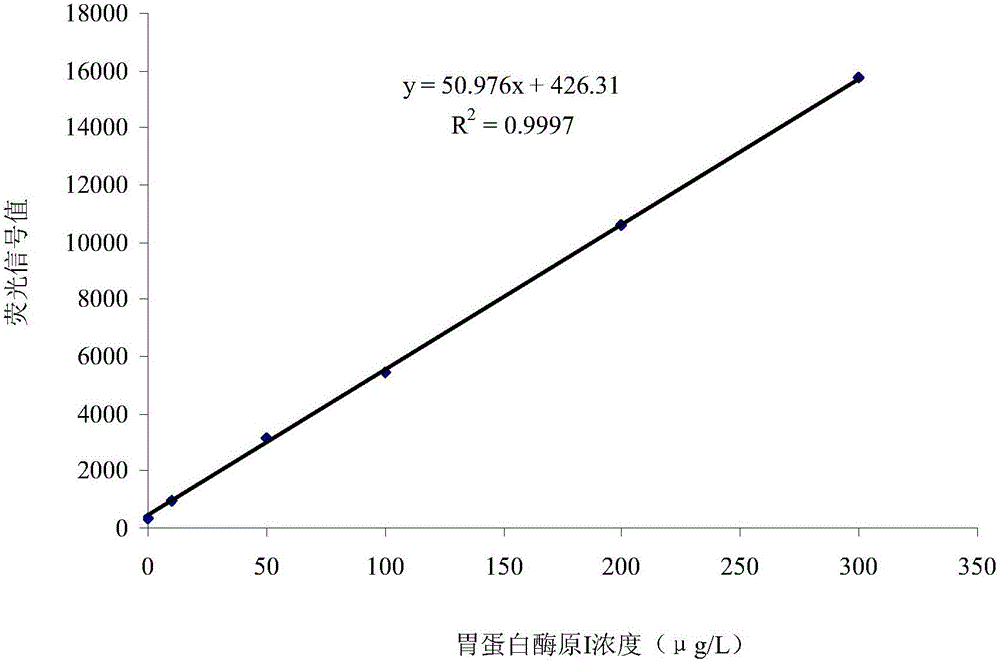
InactiveCN105785016AAvoid error accumulationReliable test resultsDisease diagnosisFluorescenceMicroparticle
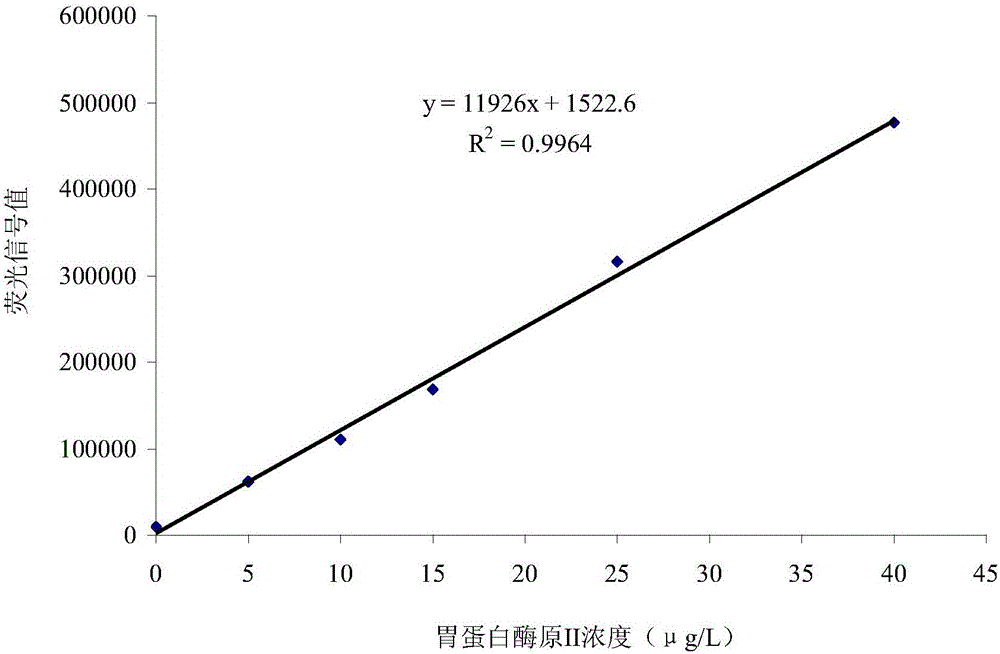
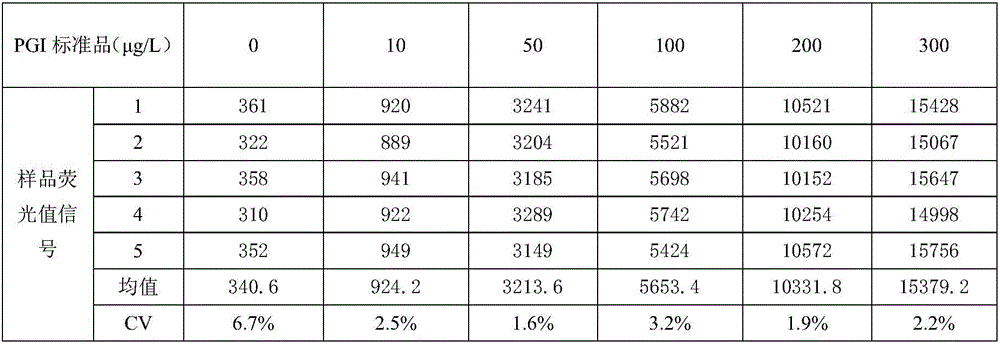
The invention relates to a double-tagging time resolution fluoroimmunoassay reagent kit based on a PG magnetic particle and a preparing method and detecting method thereof and belongs to the technical field of immune detection analyzing techniques and nanometer biological techniques. The reagent kit comprises a reaction buffer solution, a concentrated cleaning solution, an enhancing solution, a magnetic particle solution coated with PGI monoclonal antibody, a magnetic particle solution coated with PGI monoclonal antibody, a PG calibrator solution, an europium-labeled PGII monoclonal antibody solution and a samarium-labeled PGI monoclonal antibody solution which are independently packaged. The reagent kit can provide a double-tagging reaction system close to a homogeneous phase, can guarantee that the content of PGI and the content of PGII can be detected at the same time, enables a detecting result of PGI and PGII in the same sample liquid to be high in accuracy and small in error, greatly shortens reaction time, shortens detection time, improves efficiency, improves detection sensitivity and specificity and brings great convenience to clinical application.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Chromogranin A chemiluminescence immunoassay reagent kit and preparation method thereof
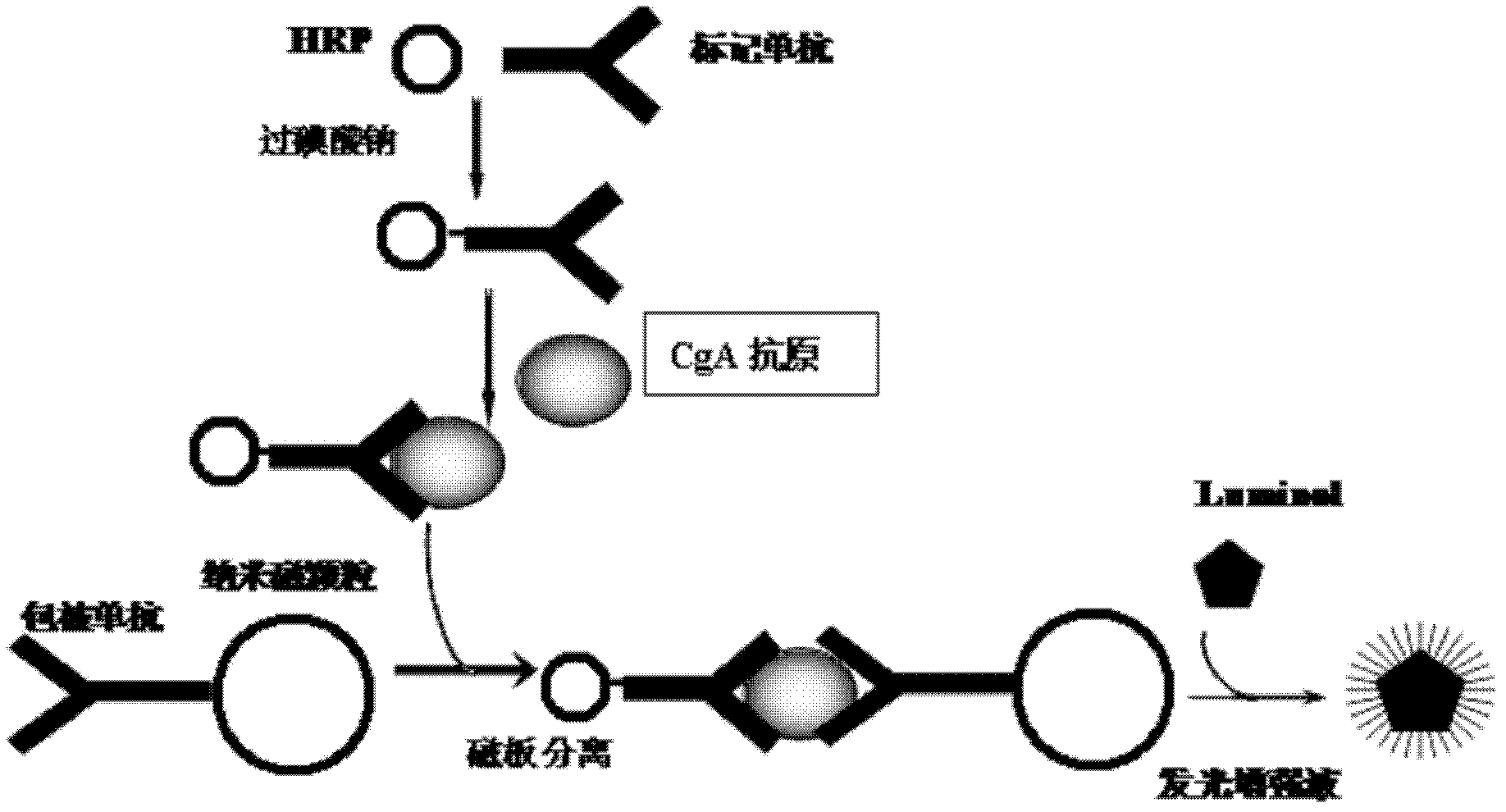
ActiveCN102520195ANo radioactive contaminationStrong specificityChemiluminescene/bioluminescenceBiological testingChromogranin AAdditive ingredient
The invention discloses a chromogranin A chemiluminescence immunoassay reagent kit and a preparation method of the reagent kit. The reagent kit comprises the following ingredients: 1) chromogranin A standard products; 2) horseradish peroxidase labeled chromogranin A monoclonal antibodies; 3) chromogranin A monoclonal antibody covered magnetic particles; 4) white non-transparent micro porous plate; 5) cleaning solution and 6) chemiluminescence substrate A and B work solution. The chemiluminescence immunoassay and the nanometer magnetic particle technology are combined, and the chromogranin A chemiluminescence immunoassay reagent kit is provided. The defects of low sensitivity and poor accuracy of the traditional enzyme-linked immunosorbent assay (ELISA) method are overcome, the radioactive pollution of the radioimmunoassay (RIA) technology is avoided, and the chromogranin A chemiluminescence immunoassay reagent kit and the preparation method thereof have the advantages that safety is realized, the environment is protected, the specificity is high, the sensitivity and the accuracy are high, and the like.
Owner:天津市协和医药科技集团有限公司
High-waterproof colorful stone-like paint
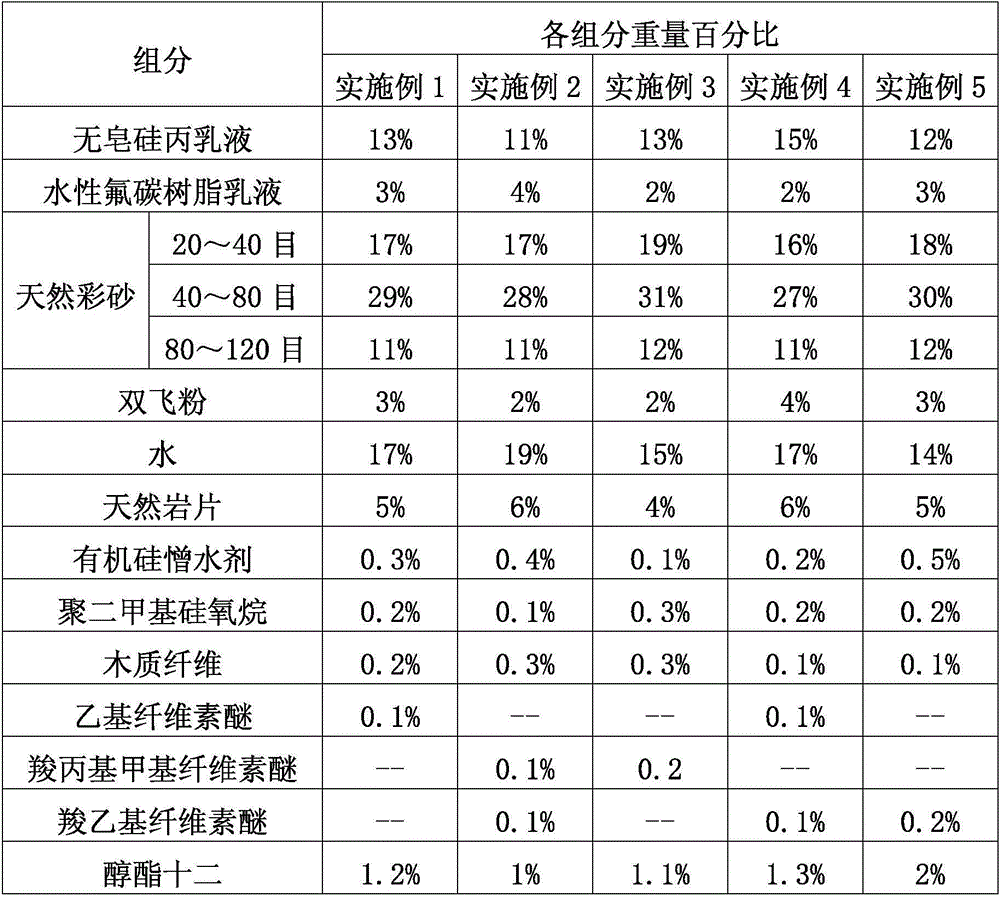
A high-waterproof colorful stone-like paint is composed of the following raw materials in percentage by weight: 11 to 15% of soap-free silicone acrylic emulsion, 2 to 4% of aqueous fluorocarbon resin emulsion, 54 to 63% of natural colorful sand, 2 to 4% of calcium carbonate and magnesium carbonate powder, 14 to 20% of water, 4 to 6% of natural rock sheets, 0.1 to 0.5% of water repellent, 0.1 to 0.3% of antifoaming agent, 0.1 to 0.3% of wood fiber, 0.1 to 0.2% of cellulose ether, and 1 to 2% of film-forming auxiliary agent. The soap-free silicone acrylic emulsion and aqueous fluorocarbon resin emulsion are mixed and used as the adhesive of the stone-like paint. Natural colorful sand with different finenesses is matched with natural rock sheet to prepare the stone-like paint. The prepared stone-like paint has the advantages of high waterproofness, natural color and luster, stone-like texture, good imitation effect, and high adhesive strength. The coating will not fade and become loosen and scratched. After drying, the paint film is compact, and moreover the paint has the advantages of strong adhesive force, large hardness, cracking resistance, alkali / acid resistance, corrosion resistance, no odor or toxicity, safety, and environment-friendliness.
Owner:黄美忠
Method for detecting content of hyaluronic acid in human serum
InactiveCN102095847ADecreased fluorescence intensityImprove bindingMaterial analysisEuropiumPollution
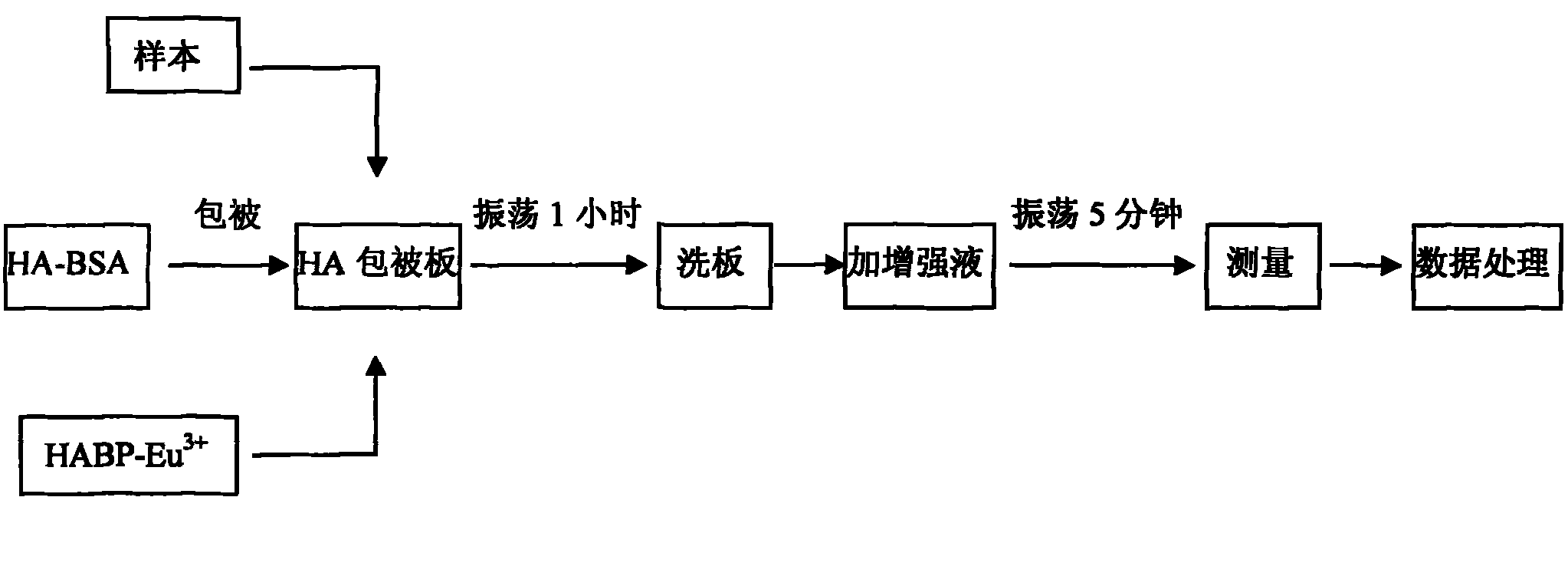
The invention relates to a method for detecting hyaluronic acid in human serum, body fluid or tissue homogenate by a radioimmunoassay method. By a method of using solid phase-coated and europium-marked hyaluronic acid binding protein (HABP-Eu<3+>), reagents such as a first antibody, a second antibody and the like are not needed, a sample is easy to add, reaction is quick, the reagents have long shelf life and are environmental-friendly, europium is stably combined with HABP, storage time can be more than 12 months, and radioactive pollution is avoided.
Owner:BEIJING NORTH INST OF BIOLOGICAL TECH
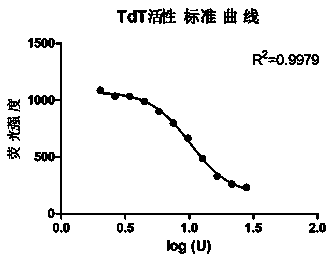
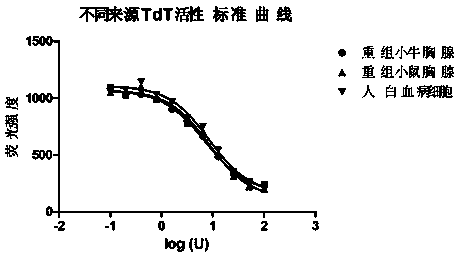
Method for measuring activity of terminal deoxynucleotidyl transferase
InactiveCN104293883ALow costEasy to operateMicrobiological testing/measurementPolymerase LSingle strand
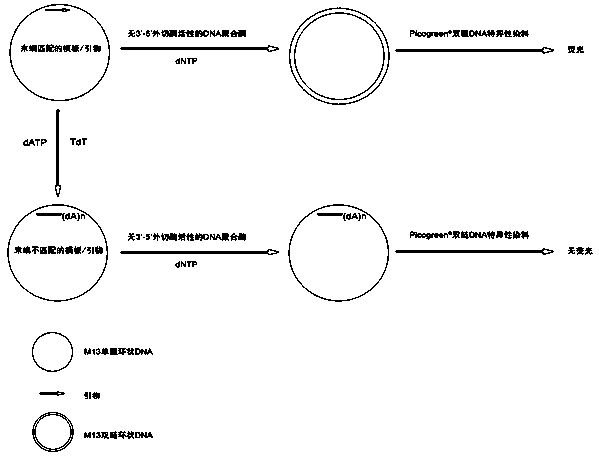
The invention discloses a method for measuring activity of terminal deoxynucleotidyl transferase. The method comprises the steps of: firstly, synthesizing a primer according to a single stranded template, annealing the single stranded template, then performing a 3' end tailing reaction catalyzed by TdT enzyme in the presence of one of four deoxyribonucleoside triphosphates, after the 3' end tailing reaction is completed, performing a polymerization reaction in the presence of enough DNA polymerase without 3'-5 'excision enzyme activity to generate double stranded DNA, finally measuring the relative quantity of the double stranded DNA or single stranded DNA in the reaction system after the polymerization reaction is finished, and deducing the activity degree of the Poly (A) polymerase according to the detection result, wherein the basic group at the first site on the used single stranded template in the 3' direction and the deoxyribonucleoside triphosphate used in the tailing reaction are not complementary. Compared with the existing method, the method disclosed by the invention is free of radioactive pollution, simple and quick to operate, low in reagent preparation cost and high in sensitivity.
Owner:VAZYME BIOTECH NANJING
PCR (polymerase chain reaction) synchronous detection kit for staphylococcus aureus enterotoxin A and B genes
InactiveCN102181547ASensitive and accurate detectionReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationNucleotideInverse polymerase chain reaction
The invention discloses a PCR (polymerase chain reaction) synchronous detection primer for staphylococcus aureus enterotoxin A and B genes, a detection kit thereof and a detection method. The nucleotide sequence of the synchronous detection primer is as shown in SEQ (sequence) ID (identity) No. 1 and 2. The invention further provides the PCR detection kit containing the primer. By adopting the detection kit, the staphylococcus aureus enterotoxin A and B in a food can be accurately and sensitively detected, and the lowest detection concentration of DNA (deoxyribonucleic acid) is 3.58ng; furthermore, the detection kit has no cross reaction with other bacteria, and the specificity is good; simultaneously, the pretreatment process of samples is simple, the consumed time is short, a large number of the samples can be detected simultaneously, and the cost is low.
Owner:BEIJING SANYUAN FOOD
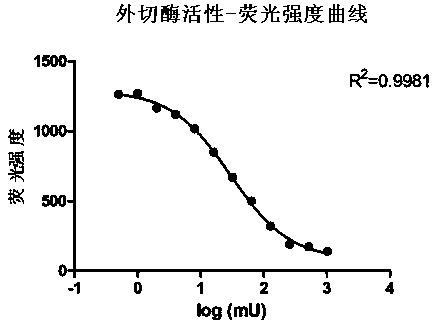
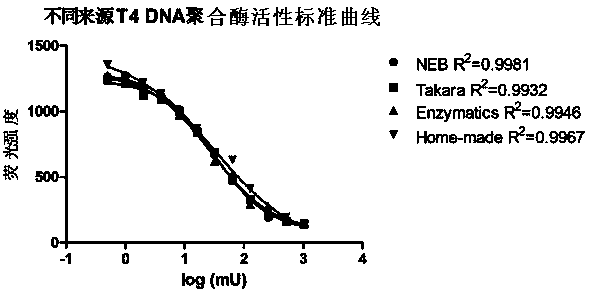
Measuring method of activity of T4 DNA polymerase
InactiveCN104313132AEasy to operateImprove throughputMicrobiological testing/measurementPolymerase LRadioactive contamination
A measuring method of activity of a T4 DNA polymerase is disclosed. The method includes: under the circumstance of no dNTP, adding the T4 DNA polymerase into a reaction system adopting a double-strand linear DNA template as a substrate, detecting the relative amount of double-strand DNA after a reaction is finished so as to measure the excision enzyme activity of the T4 DNA polymerase, and allowing the excision enzyme activity of the T4 DNA polymerase to be related to the polymerase activity so as to obtain the polymerase activity degree of the T4 DNA polymerase. The polymerase activity is measured by conveniently measuring the excision enzyme activity and by utilization of relevance between the excision enzyme activity and the polymerase activity of the T4 DNA polymerase. Compared with methods in the prior art, the method is free of radioactive contamination and simple and rapid in operation, and the method overcomes interference of the excision enzyme activity on the measurement of the polymerase activity, so that a result of the method is more accurate.
Owner:VAZYME BIOTECH NANJING
Industrial residue phosphogypsum unburned brick and producing method thereof
InactiveCN101508556AEfficient use ofReduce pollutionMixing operation control apparatusCeramic shaping apparatusGypsumRadioactive contamination
The invention provides an industrial residue phosphogypsum baking-free brick and a manufacturing method thereof. The raw material takes the phosphogypsum as the master batch and sand, sand grains and cement as accessories. The phosphogypsum is crushed, burdened, mixed and molded under the high pressure of 40MPa-85MPa to form green bricks; the product is obtained after short natural maintenance of the green bricks. The invention has the beneficial effects that the phosphorous chemical industrial residue phosphogypsum is effectively utilized so that the stacking of the phosphogypsum produces less pollution to the environment; the phosphogypsum baking-free brick saves energy and land resources and reduces emission compared with the clay sintered bricks; the brick has short production period, low cost and no radioactive pollution; the brick is a novel high quality baking-free brick which has high tensile and compressive strength and neat appearance, reaches the national JC239-2001 standard and is used for buildings.
Owner:林孝均
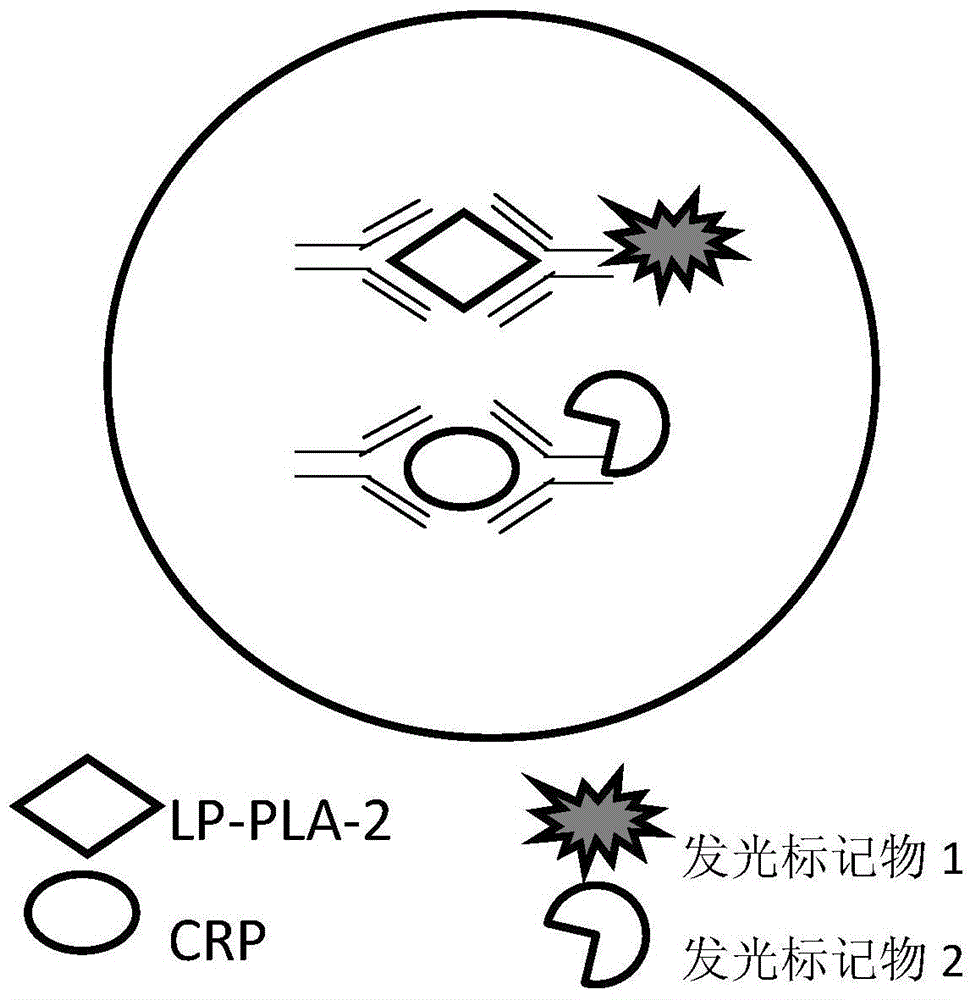
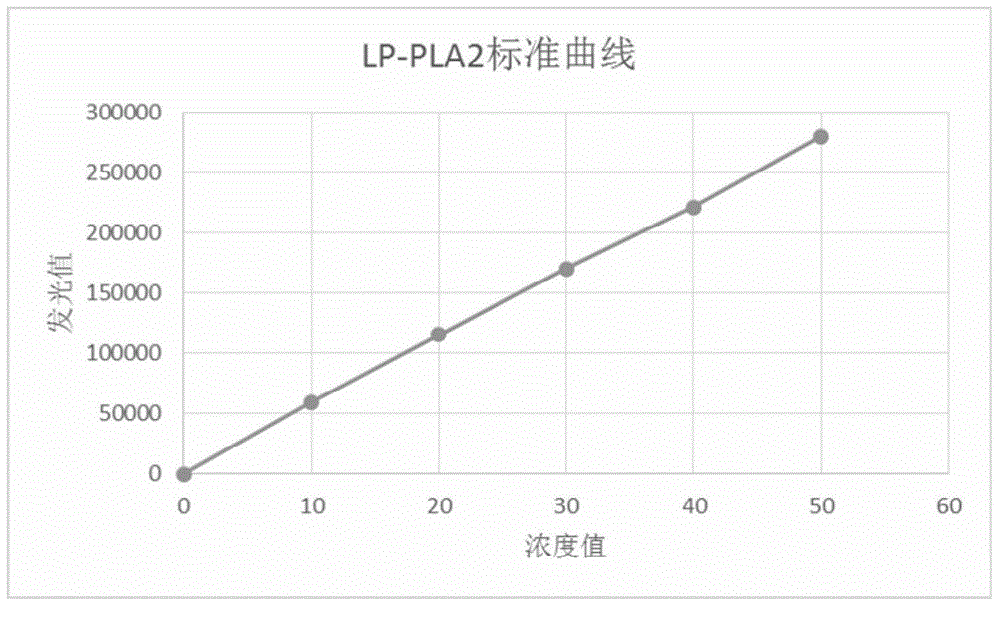
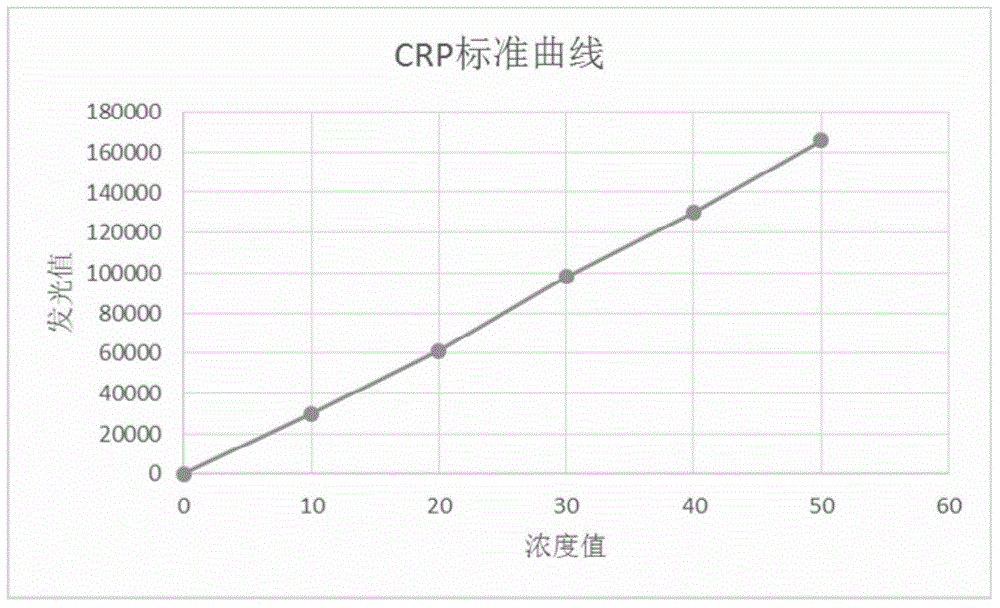
Kit of detecting the content of Lp-PLA2 and CRP on the basis of chemiluminescence, and method and application thereof
InactiveCN104820102AGuaranteed SensitivityEasy to operateBiological testingSeparation technologyMicroparticle
The invention discloses a kit of detecting the content of Lp-PLA2 and CRP on the basis of chemiluminescence, wherein the kit comprises: magnetic particles coated by an anti-fluorescein isothiocyanate polyclonal antibody, an Lp-PLA2 monoclonal antibody marked by fluorescein isothiocyanate, a CRP monoclonal antibody marked by fluorescein isothiocyanate, an Lp-PLA2 monoclonal antibody marked by alkaline phosphatase, a CRP monoclonal antibody marked by alkaline phosphatase, a chemiluminescent substrate liquid with alkaline phosphatase catalytic luminescence, a diluent liquid, a cleaning agent, an Lp-PLA2-series standard substance, and a CRP-series standard substance. The kit of detecting the content of Lp-PLA2 and CRP is used in a reaction mode of a double antibody sandwich method, so that the technical principle of chemiluminescent detection with a magnetic particle immune-separation technology is effectively utilized, by that the content of the Lp-PLA2 and the CRP in human serum or blood plasma samples are quantitatively measured and detection sensitivity is ensured. The kit is low in pre-treatment requirement on samples, is simple and reliable in the pre-treatment of the samples, can quickly detection large batches of samples in high throughput and is convenient to operate and produce.
Owner:南京格耀生物科技有限公司
Quantitative gastrin-releasing peptide precursor kit, as well as preparation method and detection method thereof
InactiveCN104089949ALow costHigh sensitivityChemiluminescene/bioluminescenceQuality controlTrue positive rate
The invention relates to a quantitative gastrin-releasing peptide precursor (ProGRP) kit and a detection method thereof. A method comprises seven steps of preparing a magnetic separation reagent, preparing an enzyme reactant, preparing a reaction enhancer, preparing a calibration substance diluent, preparing a calibration substance and a quality control substance, preparing a cleaning concentrate liquid and preparing a substrate solution. According to the quantitative gastrin-releasing peptide precursor kit and the detection method thereof, the sensitivity and the specificity are relatively high, the detection result obtaining time is relatively short, the operation mode is relatively simple, cell lung cancers (SCLC) and non small cell lung cancer (NSCLC) can be distinguished, and the kit has the important significance on early diagnosis on lung cancers.
Owner:JIANGSU FLON BIOTECH
Chemical luminescence immune assay determination reagent kit for prostate gland acid phosphatase and preparation method thereof
InactiveCN101368962AGuaranteed SensitivityReduce use costChemiluminescene/bioluminescenceMonoclonal antibodyProstatic acid phosphatase
The invention provides a chemiluminescence immunoassay test kit of prostatic acid phosphatase, which belongs to the clinical blood detecting and assaying technique field. The test kit includes: 1) prostatic acid phosphatase calibrating article; 2) solid-phase carrier which is coated by monoclonal antibody against prostatic acid phosphatase; 3) monoclonal antibody against prostatic acid phosphatase which is labeled by enzyme; 4) chemiluminescence zymolyte on which the enzyme reacts; 5) washing liquid. Furthermore, the invention also provides a preparing method of the test kit which includes steps as following: the calibrating article is prepared; the solid-phase carrier is coated by the monoclonal antibody against prostatic acid phosphatase; the monoclonal antibody against prostatic acid phosphatase is labeled by the enzyme; the calibrating article, the enzyme labeled antibody and the chemiluminescence zymolyte above can be filled and packed through separate packing; and the finished produce is prepared after assembly. With security and reliability, the test kit has the advantages that the sensitivity is high, and the specificity is strong.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Ready-mixed plastering mortar for interior wall and production method of ready-mixed plastering mortar
The invention relates to a ready-mixed plastering mortar for an interior wall. The ready-mixed plastering mortar for the interior wall is characterized by being prepared from the following raw materials: 60-70 parts of artificial sand, 12-13 parts of cement, 10-12 parts of II grade coal ash, 2-3 parts of cellulose ether, 2-3 parts of redispersible powder and 0.1-0.5 part of additives. The ready-mixed plastering mortar for the interior wall has excellent heating retaining property and water retaining property, and the adopted additives are capable of remarkably improving the properties such as anti-permeability, crack resistance and shrinkage reduction property of the mortar.
Owner:GUANGXI HENGXIAN HENGFENG BUILDING MATERIAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com