Double-tagging time resolution fluoroimmunoassay reagent kit based on PG magnetic particle
A time-resolved fluorescence and immunoassay technology, applied in the field of immunoassay analysis technology and nanobiology, can solve the problems of large error of PGI/PGII ratio, low efficiency, unsuitable for sample screening, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
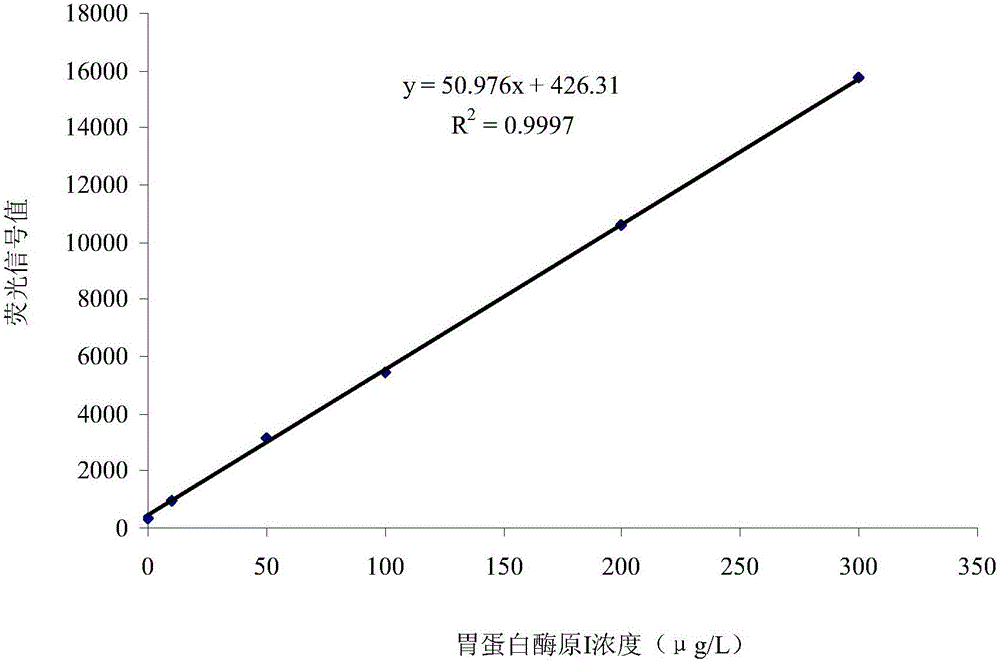
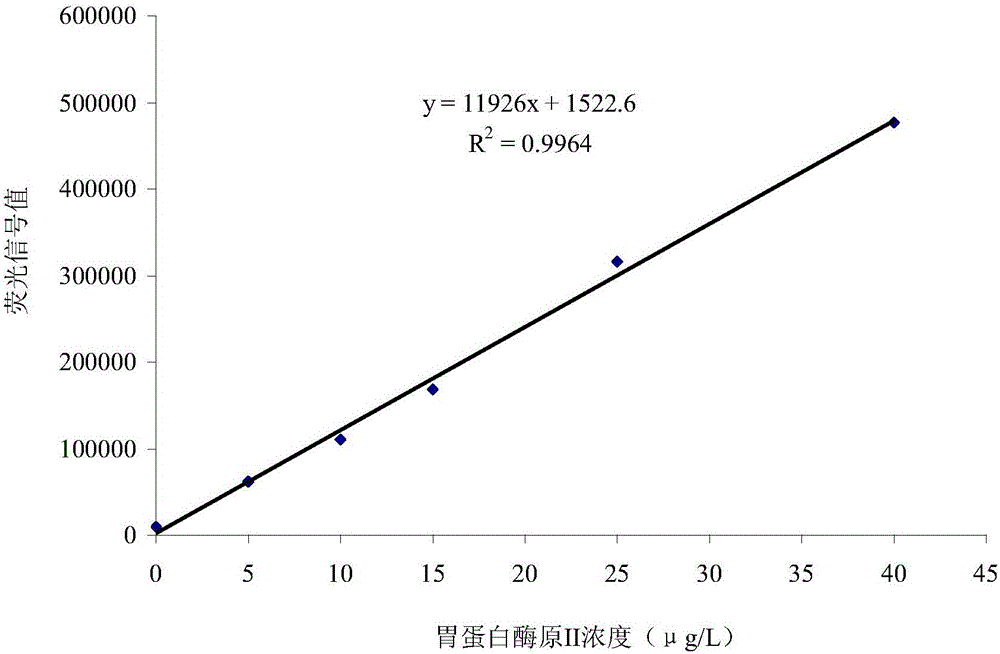
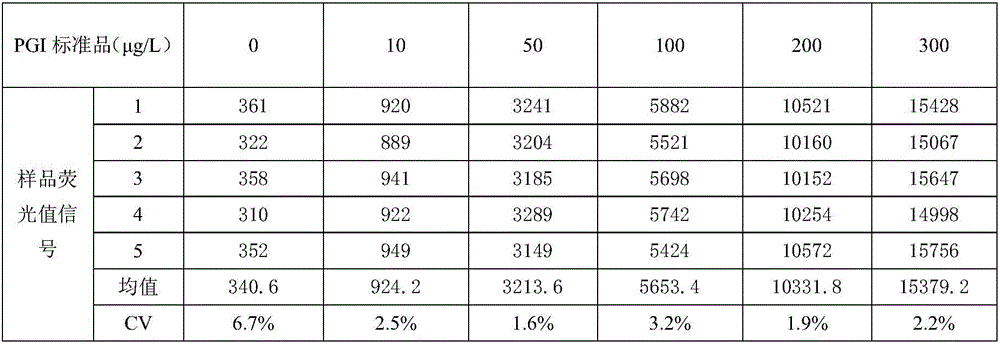
[0075] This embodiment provides a dual-labeled time-resolved fluorescence immunoassay kit based on PG magnetic particles, including individually packaged reaction buffer, concentrated cleaning solution, enhancement solution, magnetic particle solution coated with PGI monoclonal antibody, and package Magnetic particle solution of PGII monoclonal antibody, PG calibrator solution, europium-labeled PGII monoclonal antibody solution, and samarium-labeled PGI monoclonal antibody solution, where:
[0076] The europium-labeled PGII monoclonal antibody solution is Eu 3+ -N 2 -[P-isocyanic acid-benzyl]-diethylenetriaminetetraacetate-labeled PGII monoclonal antibody is prepared, and the PGII monoclonal antibody is compatible with the Eu 3+ -N 2 -The mass ratio of [P-isocyanate-benzyl]-diethylenetriaminetetraacetate sodium is 5:1;
[0077] The samarium-labeled PGI monoclonal antibody solution is Sm 3+ -N 2 -[P-Isocyanic acid-benzyl]-diethylenetriaminetetraacetate labeled PGI monoclonal antibody...
Embodiment 2
[0103] This embodiment provides a dual-labeled time-resolved fluorescence immunoassay kit based on PG magnetic particles, including individually packaged reaction buffer, concentrated cleaning solution, enhancement solution, magnetic particle solution coated with PGI monoclonal antibody, and package Magnetic particle solution of PGII monoclonal antibody, PG calibrator solution, europium-labeled PGII monoclonal antibody solution, and samarium-labeled PGI monoclonal antibody solution, where:
[0104] The europium-labeled PGII monoclonal antibody solution is Eu 3+ -N 2 -[P-isocyanic acid-benzyl]-diethylenetriaminetetraacetate-labeled PGII monoclonal antibody is prepared, and the PGII monoclonal antibody is compatible with the Eu 3+ -N 2 -The mass ratio of [P-isocyanate-benzyl]-diethylenetriaminetetraacetate sodium is 5:1;
[0105] The samarium-labeled PGI monoclonal antibody solution is Sm 3+ -N 2 -[P-Isocyanic acid-benzyl]-diethylenetriaminetetraacetate labeled PGI monoclonal antibody...
Embodiment 3
[0131] This embodiment provides a dual-labeled time-resolved fluorescence immunoassay kit based on PG magnetic particles, including individually packaged reaction buffer, concentrated cleaning solution, enhancement solution, magnetic particle solution coated with PGI monoclonal antibody, and package Magnetic particle solution of PGII monoclonal antibody, PG calibrator solution, europium-labeled PGII monoclonal antibody solution, and samarium-labeled PGI monoclonal antibody solution, where:
[0132] The europium-labeled PGII monoclonal antibody solution is Eu 3+ -N 2 -[P-isocyanic acid-benzyl]-diethylenetriaminetetraacetate-labeled PGII monoclonal antibody is prepared, and the PGII monoclonal antibody is compatible with the Eu 3+ -N 2 -The mass ratio of [P-isocyanate-benzyl]-diethylenetriaminetetraacetate sodium is 5:1;
[0133] The samarium-labeled PGI monoclonal antibody solution is Sm 3+ -N 2 -[P-Isocyanic acid-benzyl]-diethylenetriaminetetraacetate labeled PGI monoclonal antibody...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com