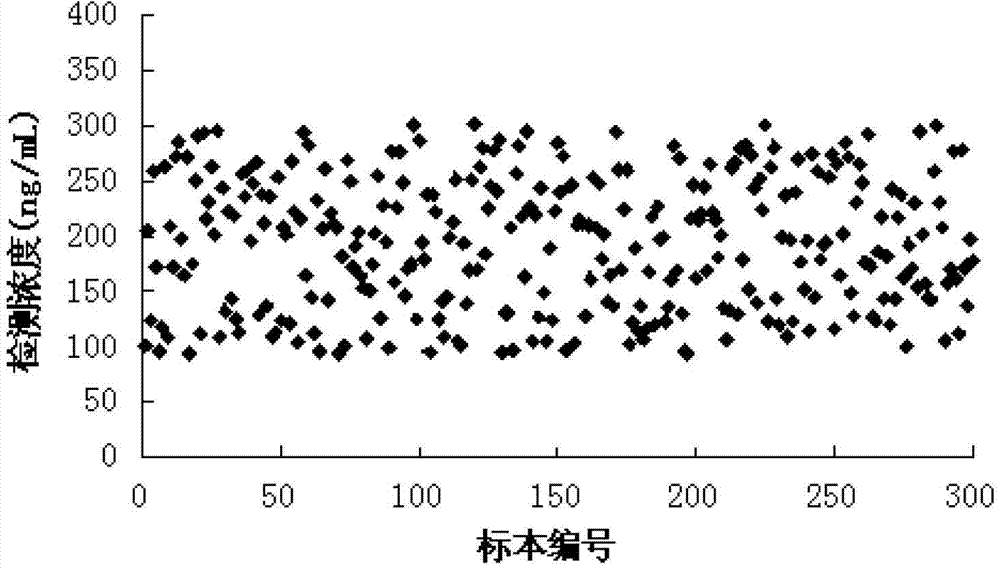
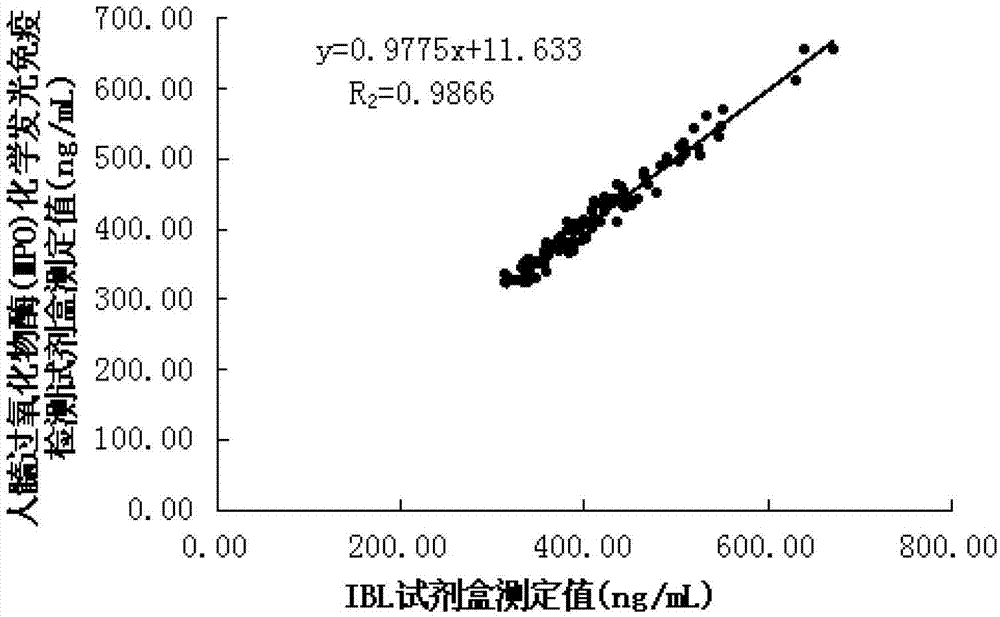
Human myeloperoxidase chemiluminescent immunodetection kit
A myeloperoxidase and chemiluminescence immunological technology, applied in the field of immunoassay medicine, to achieve the effects of good accuracy, improved sensitivity and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A human myeloperoxidase chemiluminescent immunoassay kit, comprising the following components:
[0033] 1) Human myeloperoxidase standard;
[0034] 2) Microwell plate coated with human myeloperoxidase monoclonal antibody;
[0035] 3) Alkaline phosphatase-labeled human myeloperoxidase monoclonal antibody;
[0036] 4) The chemiluminescent substrate AMPPD that the above enzyme acts on;
[0037] 5) Concentrated washing liquid;
[0038] 6) Composition of semi-finished products and finished products;
[0039] in:
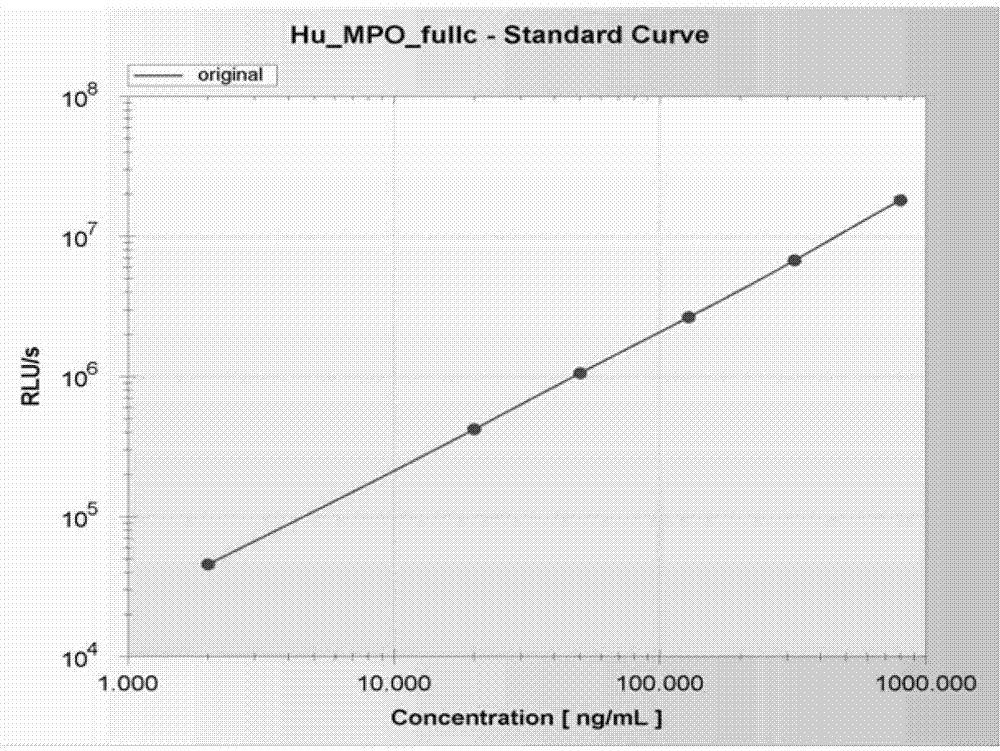
[0040] 1. The human myeloperoxidase standard product is prepared by the following method: mix 0.02mol / L phosphate buffer solution with a pH value of 7.4 and fetal bovine serum in a volume ratio of 4:1 to prepare a basic buffer solution, and use Human myeloperoxidase was diluted to concentrations of 0, 25, 64, 160, 400, and 1000 ng / mL in basic buffer solution.
[0041] 2. The preparation steps of the microwell plate coated with human myeloperoxidase monoclon...
Embodiment 2
[0067] A human myeloperoxidase chemiluminescent immunoassay kit, comprising the following components:
[0068] 1. Human myeloperoxidase standard substance (same as Example 1)
[0069] 2. Microwell plate coated with human myeloperoxidase monoclonal antibody (same as Example 1)
[0070] 3. Horseradish peroxidase-conjugated monoclonal antibody to human myeloperoxidase
[0071] Dissolve 4 mg of horseradish peroxidase (HRP) in 1 mL of deionized water, add 0.4 mL of 50 mM sodium periodate aqueous solution, shake at room temperature for 30 min, dialyze overnight with 1 mM sodium acetate buffer (pH 4.4), add 6 mg of MPO monoclonal antibody, shake overnight at 2-8°C, reduce with 400 μL of 200 mM NaBH4, then dialyze through 0.02M PBS with a pH value of 7.4 overnight, use HPLC for secondary purification, collect protein peaks and add an equal volume Glycerol, stored at -20°C.
[0072] 4. The chemiluminescent substrate luminol acted by the above enzymes
[0073] The formula of describ...
Embodiment 3
[0088] Embodiment 3 The using method of kit of the present invention
[0089] The use of the human MPO chemiluminescent immunoassay kit prepared in Example 1, the specific operations are as follows:
[0090] 1) Take out the kit of the present invention from the refrigerator at 4°C, and equilibrate to room temperature;
[0091] 2) Take a microwell plate prepared in Step 2 of Example 1 and number it, and repeat all experiments with double holes;
[0092] 3) Add 25 μL each of the calibrator and the sample to be tested at a concentration of 0, 25, 64, 160, 400, and 1000 ng / mL into the wells of the microplate, and set a blank well for each experiment, and then remove the blank well Add 100 μL of the enzyme-labeled human myeloperoxidase monoclonal antibody prepared in Step 3 of Example 1 to each well;
[0093] 4) Oscillate on the oscillator for 30s to mix;
[0094] 5) Seal the plate with parafilm and incubate at 37°C for 45 minutes;
[0095] 6) Shake off the reaction solution, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ph value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com