Application of cleavable polyethylene glycol (PEG) lipid derivative to preparation
A technology of lipid derivatives and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0112] Example 1-1 Changes in plasma clearance of rats with repeated injections of PEG-CHM modified liposomes
[0113] In the liposome formulation, PEG-DSPE was replaced with PEG-CHM, and the preparation of PEG-CHM modified liposome was the same as "Comparative Example 1".
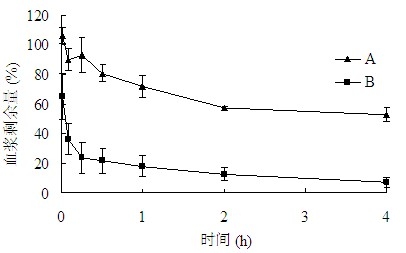
[0114] Take Wista rats with a body weight of 250-300 g, divide them into 2 groups, 3 rats in each group, and administer them by tail vein injection according to Table 3, and the rest of the operations are the same as in "Example 1". see results image 3 , the results showed that the remaining amount of calcein plasma at each time point in group C was slightly higher than that in group D (P0.1), indicating that only a slight accelerated blood clearance occurred.
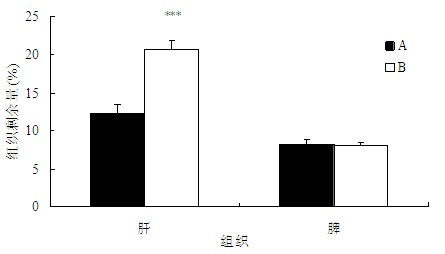
[0115] After the second tail vein injection of PEG-CHM modified liposomes in groups C and D, the tissue distribution at 4 h was shown in Fig. Figure 4 . Compared with group C, the amount of hepatic aggregation in group D increased (P0.1).
[...
Embodiment 1-2
[0122] Example 1-2 Changes in plasma clearance of rats with repeated injections of PEG-CHS modified liposomes
[0123] The PEG-DSPE in the liposome formulation was replaced with PEG-CHS, and the preparation of the PEG-CHS modified liposome was the same as in "Comparative Example 1".
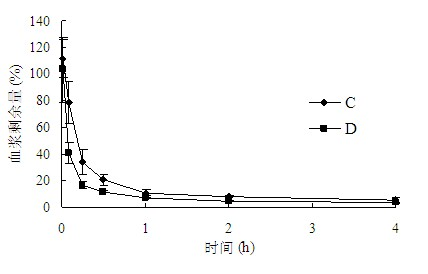
[0124] Take Wista rats with a body weight of 250-300 g, divide them into 2 groups, 3 rats in each group, and administer them by tail vein injection according to Table 5, and the remaining operations are the same as in "Example 1". see results Figure 5 , the results showed that the clearance curves of E and F groups were almost identical. The pharmacokinetic parameters were calculated according to the non-compartment model, and the results are shown in Table 6. There was no significant difference in each pharmacokinetic parameter (P>0.1). It shows that no ABC phenomenon occurs.
[0125] After the second tail vein injection of PEG-CHS modified liposomes in groups E and F, the tissue distributi...
Embodiment 2-1
[0142] Example 2-1 Changes in plasma clearance of rats with repeated injections of PEG-CHM modified vesicles
[0143] The PEG-CHOL in the vesicle prescription was replaced with PEG-CHM, and the preparation of PEG-CHM modified CHST vesicles was the same as in "Comparative Example 2".
[0144] Take Wista rats with a body weight of 250-300 g, divide them into 2 groups, 3 rats in each group, and administer them by tail vein injection according to Table 9, and the remaining operations are the same as in "Example 1". see results Figure 9 , Table 10. The results showed that the remaining amount of calcein at several time points at the beginning of the curve of group I was slightly higher than that of group J, and by comparing the pharmacokinetic parameters, it can be seen that although the half-life is slightly reduced, there is no statistical difference between the pharmacokinetic parameters. There were no significant differences (P>0.1), indicating that only a slight ABC phenome...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com