Application method of tumor cell-derived exosome antigen in DC vaccine
A tumor cell and tumor vaccine technology, which is applied in the application field of tumor cell-derived exosome antigens in DC vaccines, can solve the problems of low direct uptake efficiency of antigens, etc., to enhance the body's anti-tumor immunity, enhance cytotoxic effect, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
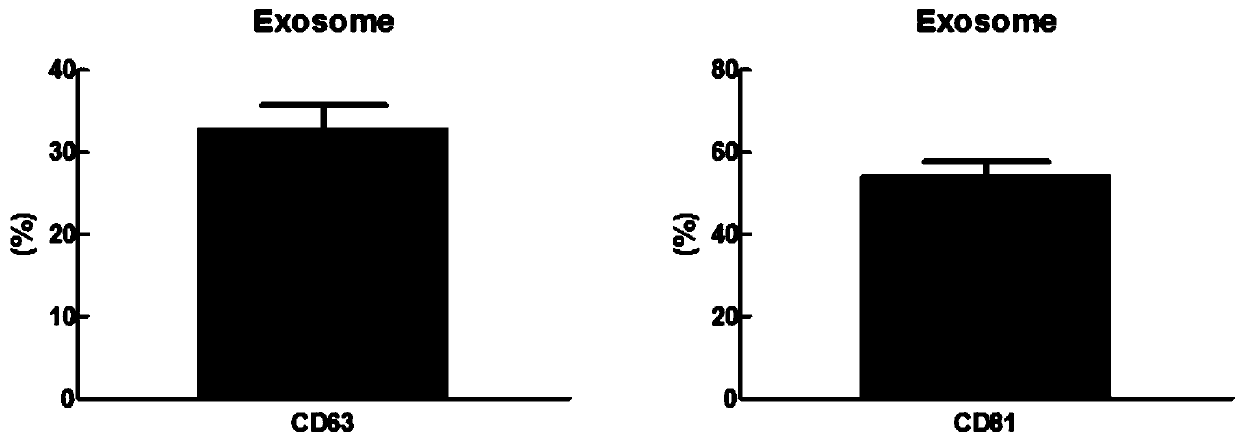
[0057] Example 1, Preparation of Exosomes in the Culture Supernatant of Lung Tumor A549
[0058] 1. Preparation of exosome-free FBS
[0059] Dissolved and inactivated FBS (Gibco, USA) was centrifuged at 12 000 g at 4°C for 30 min to remove impurities with larger molecular weights. Use a syringe to slowly inject the serum supernatant into the ultracentrifuge tube, and remember not to generate air bubbles. After the supernatant is added to the bottleneck of the centrifuge tube, use an electric fuser to melt the centrifuge tube mouth. The centrifuge tubes that have been melted are placed diagonally and symmetrically on an ultracentrifuge, and centrifuged at 150,000 g for 12 hours at 4°C. After centrifugation, cut off the seal, aspirate the supernatant, and filter the supernatant with a 0.22 μm syringe filter.
[0060] The filtered supernatant (FBS with exosomes removed) was subpackaged and stored at -20°C for future use.
[0061] 2. Obtain tumor cell culture supernatant in ser...
Embodiment 2
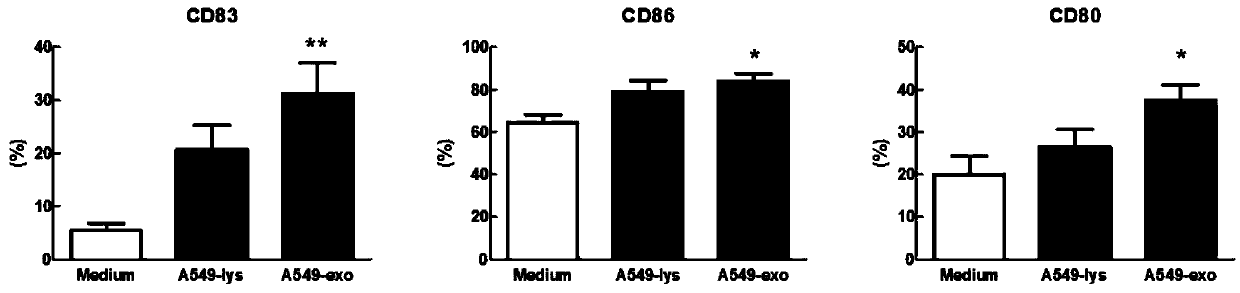
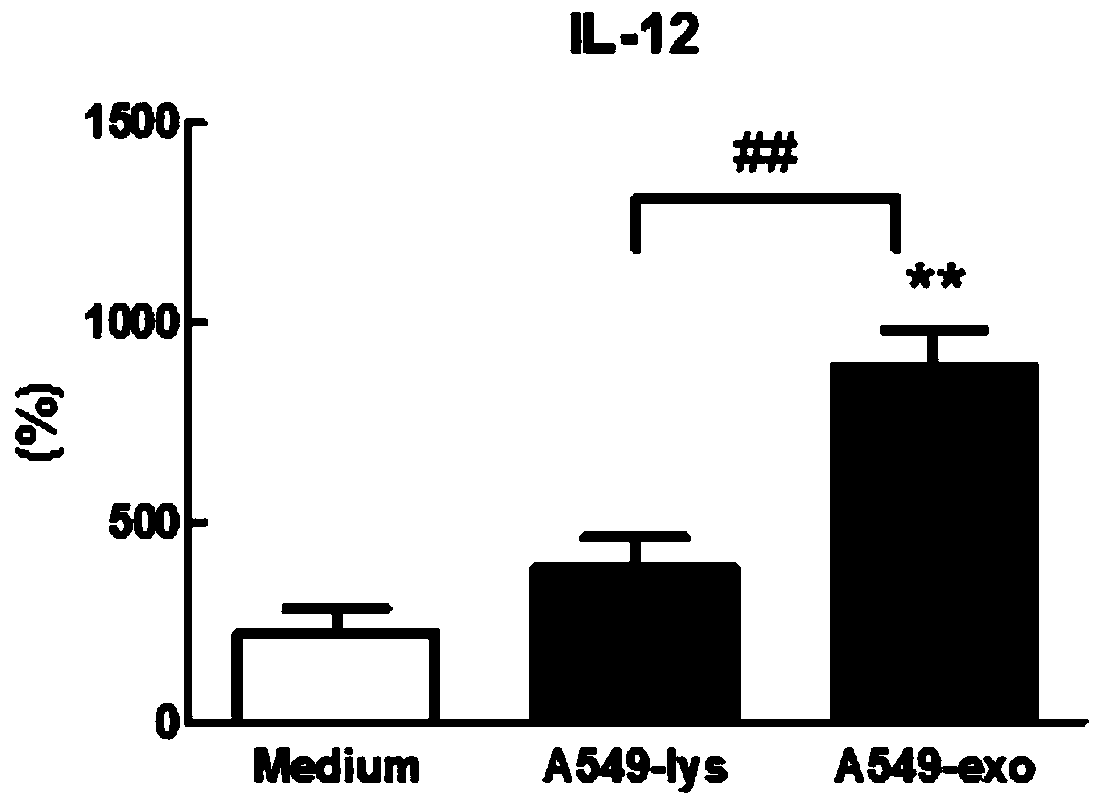
[0080] Example 2. Application of exosomes in tumor cell culture supernatant in DC vaccine
[0081] 1. A549 cell culture supernatant-derived exosomes activate DC
[0082] 1. Induction and culture of human peripheral blood-derived dendritic cells (DC)
[0083] (1) Collect 25ml of peripheral blood from healthy volunteers in vitro, pipette the peripheral blood up and down to mix, 800g, centrifuge for 5min, the upper layer is light yellow plasma layer, and the remaining liquid is concentrated blood cells.
[0084] (2) Put the upper layer of plasma obtained in step (1) into a centrifuge tube and seal it with a parafilm, inactivate it in a water bath at 56°C for 30 minutes, and take the supernatant after centrifugation for later use.
[0085] (3) Aspirate the blood cells obtained in step (1) and slowly transfer the diluted blood cells into a centrifuge tube filled with lymphocyte separation medium (GE, USA) at 1 cm from the slope of the separation medium. The transferred blood cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com