Vaccine composition as well as preparation method and application thereof
A vaccine composition and a technology for chicken Newcastle disease virus, applied in the field of vaccine compositions, can solve the problems of increased mortality, slow development, economic loss and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 The preparation of chicken Newcastle disease, infectious bronchitis virus liquid and mycoplasma synoviae bursae bacterial liquid
[0039] 1. Selection of virus strains
[0040] The LaSota strain of the Newcastle disease virus and the M41 strain of the infectious bronchitis virus were used to manufacture this product, both of which were purchased from the China Veterinary Drug Control Institute. Mycoplasma synovia was isolated and identified by the laboratory itself, and it was HN1 strain.
[0041] 2. Virus Fluid Preparation
[0042] 2.1 Preparation of virus solution for chicken Newcastle disease virus La Sota strain vaccine
[0043] 2.1.1 Inoculation
[0044] Take the virus seeds for production and dilute to 10 with sterile physiological saline -3 , Inoculate 10-day-old susceptible chicken embryos through the allantoic cavity, 0.1ml per embryo, seal the pinhole after inoculation, and continue incubation at 36-37°C without turning the eggs.
[0045] 2.1.2 ...
Embodiment 2
[0079] The preparation of embodiment 2 vaccine
[0080] 1 oil phase preparation
[0081] Take 94 parts of white oil for injection, add Siben-806 parts after mixing, add 2 parts of aluminum stearate, add and stir until transparent, autoclave for later use, which is the oil phase.
[0082] 2 Aqueous phase preparation
[0083] Mix the antigens in a sterile container in proportion, add 4% sterilized and cooled Tween-80, and stir while adding, until the Tween-80 is completely dissolved, which is the water phase.
[0084] 3 emulsification
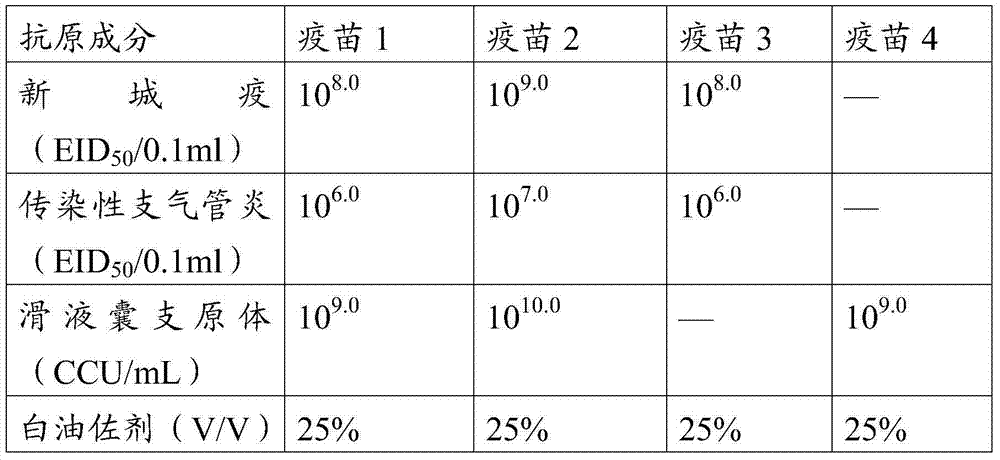
[0085] Take 3 parts of the oil phase and put them in the oil phase tank, start the motor to stir, then slowly add 1 part of the water phase, transfer to the emulsification tank after adding, and then emulsify at 2800r / min for 40 minutes. Add 1% thimerosal before the emulsification is terminated to make the final concentration 0.01%. The specific formula of the vaccine is shown in the table below.
[0086] Table 1 Vaccine composition and cont...
Embodiment 3
[0116] Embodiment 3 Newcastle disease, infectious bronchitis, mycoplasma synovialis triple inactivated vaccine and Newcastle disease, infectious bronchitis double inactivated vaccine+mycoplasma synovialis inactivated vaccine immune side reaction comparison
[0117] Grouping and immunization of 1SPF chickens
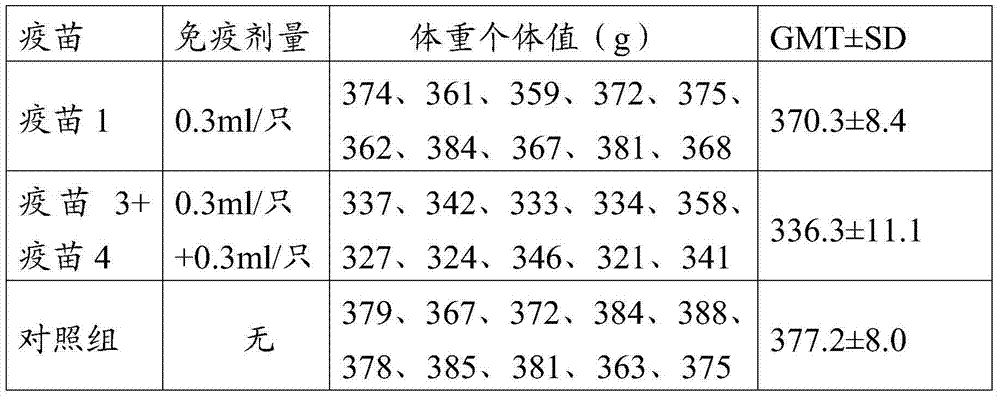
[0118] Get 20 SPF chickens of age of 14 days, wherein 10 vaccine 1, 0.3ml / of subcutaneous immunization embodiment 2 preparations, other 10 vaccine 3 (0.3ml / ) and vaccine 4 (0.3ml / ) of subcutaneous immunization embodiment 2 preparations simultaneously 0.3ml / piece). In addition, 10 SPF chickens of the same age were not immunized as controls.
[0119] 2 side effects comparison
[0120] After 14 days of immunization, all chickens were weighed, and the body weight results are shown in Table 6. The results showed that, compared with the control group, the triple vaccine immunization group had no significant difference in body weight at 14 days after injection (ANOVA, P>0.05)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com