Preparation method of mycoplasma synoviae monoclonal antibody
A technology of Mycoplasma synovialis and monoclonal antibody, which is applied in the directions of microorganism-based methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve the problem of inability to produce an immune response to the whole Mycoplasma bacteria.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of antigen
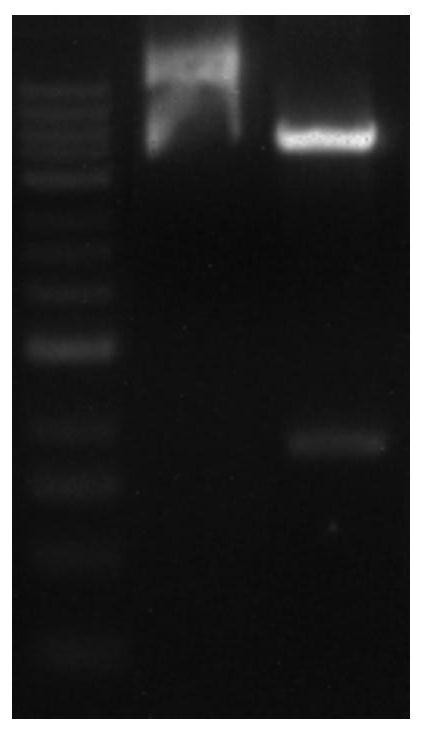
[0027] (1) Insert the P20 gene and P13 gene into the expression vector PET-32a to obtain the recombinant expression vector, transform the Escherichia coli competent cell DH5α with the recombinant expression vector, and screen out the positive clone recombinant plasmid after enzyme digestion, PCR identification and sequencing PET-32a-P20-P13; after BamHI / / XhoI digestion and electrophoresis detection of recombinant plasmid PET-32a-P20-P13 figure 1 As shown, the P20 gene sequence is shown in the sequence listing SEQ ID NO: 1, and the P13 gene is shown in the sequence listing SEQ ID NO: 2; the gene sequence of the PET-32a-P20-P13 recombinant protein is shown in the sequence listing SEQ ID NO: 2. ID NO:3 shown.
[0028] The amino acid sequence of the protein encoded by the P20 gene is shown in the sequence listing SEQ ID NO: 4, and the amino acid sequence of the protein encoded by the P13 gene is shown in the sequence listing SEQ I...
Embodiment 2
[0031] Example 2: Mouse immunization, cell fusion and subcloning
[0032] 1) For the first immunization, Freund's complete adjuvant and an equal amount of recombinant protein were used to emulsify, and after emulsification, 5 6-8 week-old Balb / c female mice were subcutaneously inoculated, and each mouse was inoculated with a dose of 200ug of recombinant protein. After that, immunize once every two weeks. After the second immunization, the adjuvant was replaced by Freund's incomplete adjuvant. After three immunizations, blood was collected from the tail vein at intervals of one week to detect antibodies, and the inactivated whole bacteria of Mycoplasma synovium was used as the coating antigen. Perform antibody testing.
[0033] 2) The mouse with the highest antibody titer was selected as the mouse for cell fusion, and the recombinant protein was inoculated intraperitoneally before the fusion for booster immunization once.
[0034] 3) Preparation of myeloma cells: Select SP2 / 0 ...
Embodiment 3
[0039] Example 3: Preparation and verification of antibodies
[0040] 1) Antibody production: The five cell lines obtained above were successively scaled up and cultured to gradually realize large-scale production of antibodies. The process is as follows, first recover in a 24-well cell plate, add feeder cells to promote the rapid growth of the hybridoma cell line during recovery, transfer the cells to a T25 cell culture flask for culture when the growth reaches 75% of the bottom of the well, and add feeder cells at the same time . When the T25 cell culture flask grew to 75% of the bottom area of the flask, the hybridoma cells were transferred to the T75 cell culture flask, then to the T225 cell culture flask, and then transferred to a 10-liter spinner bottle for culture. The titer of the antibody was detected by retaining samples at each stage to determine the genetic stability of the cells. The results of the titer determination are shown in Table 1:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com