Stilbene Estrogen Nucleic Aptamer and Its Application
A stilbene, nucleic acid aptamer technology, applied in biochemical equipment and methods, material testing products, instruments, etc., can solve the problems of time-consuming, labor-intensive, production, transportation, storage constraints, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0036] Example 1 Screening of stilbene estrogen DNA aptamers
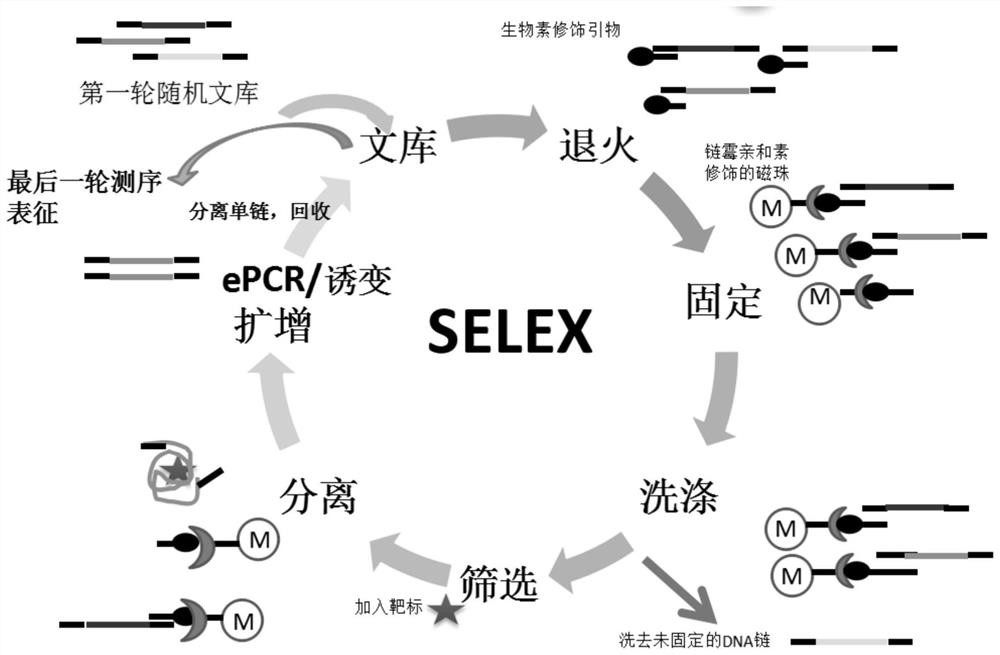
[0037] The SELEX process of this embodiment is shown in figure 1 , using streptavidin-modified magnetic beads as the stationary phase, using immobilized libraries and target elution for screening.
[0038] 1. Immobilization of nucleic acid library and streptavidin-modified magnetic beads
[0039] In the first round of screening, take the primary library lib dry powder 1OD (sequence information: 5'-ttcagcactccacgcatagc(SEQ ID NO.04)-n40-cctatgcgtgctaccgtgaa(SEQ ID NO.05)-3') synthesized from TaKaRa Company, and put it into Centrifuge at 12000rpm / min, centrifuge for 10min, then add 260μL DPBS (0.9mMCaCl 2 , 2.7mM KCl, 0.5mM MgCl 2 6H2O, 0.137M NaCl, 1.1mM KH 2 PO4, 8.1mM Na 2 HPO 4 ) dissolved. After dissolving, shake with a vortex for about 1 min, and then centrifuge at 12000 rpm / min for 10 min to prepare a 5 μM library solution. Then take 5nmol of bio-P3 primer dry powder synthesized from GenScript, centrif...
Embodiment 2
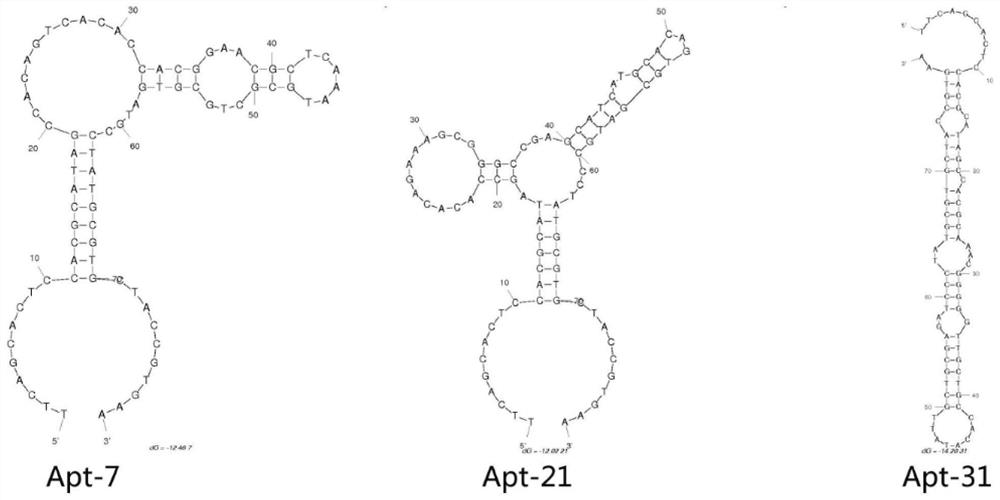
[0051] Example 2 Cloning and sequencing of nucleic acid aptamers and prediction of secondary structure of candidate aptamers
[0052] 1. Recovery of PCR products
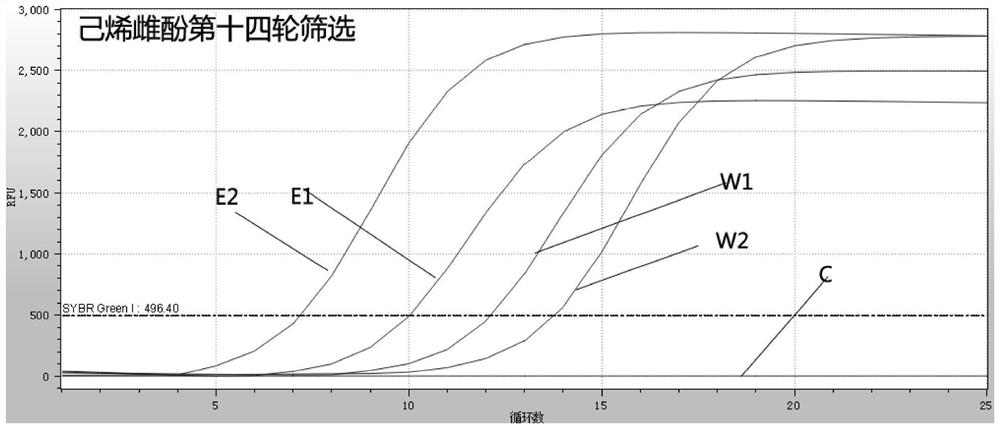
[0053] Using non-modified upstream and downstream primers, the 14th round of screening ( figure 2 ) to amplify the secondary library obtained, followed by separation on 3% agarose gel, cutting the gel to recover the target band, and using the Axygan brand agarose gel recovery kit to recover the target product.
[0054] 2. Ligation and transformation of ligation products
[0055] 1) Add 1 μL full gold pEASY-T5 Zero vector and 4 μL aptamer PCR product into a microcentrifuge tube;
[0056] 2) Ligate at 25°C for 10 minutes (the pEASY-T5 Zero vector kit comes with a ligase);
[0057] 3) Add the ligated product to 50 μL DH5α competent cells, and place in ice for 20 minutes;
[0058] 4) After heat shock at 42°C for 30s, place on ice for 2min;
[0059] 5) Add 250 μL of LB medium (without resistance) preheated at 37°C,...
Embodiment 3
[0064] Example 3: Specific detection of nucleic acid aptamers and stilbene estrogens
[0065] 1. Detection specificity of gold nanometer colorimetric method
[0066] 1) Preparation of nano-gold: set the total volume in one tube of this example to 400 μL (total system), add 100 μL of prepared nano-gold to a 1.5mL centrifuge tube, add 200 μL ddH 2 O;
[0067] 2) Add aptamer to protect gold nanoparticles: then add aptamer Apt-7 with a final concentration of 300nM (that is, add 12 μL of 10 μM DPBS aptamer to it), take the above tube on a circular mixer at 10r / min, and incubate at room temperature for 20min ;
[0068] 3) Add target: 40 μL of 10 mM diethylstilbestrol (DES), hexestrol (HEX), dienestrol (DIES), estrone (E1) and β-estradiol were sequentially added to tubes 1-5 of the experimental group (E2) solution (the final concentration of the target solution and the analog solution is 1000 μM; add 40 μL of matrix solution to tube 0 of the control group;
[0069] 4) Take the ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com