Intracellular immunity
A technology of immunoglobulins and compounds, applied in the direction of immunoglobulins, antiviral immunoglobulins, antibacterial drugs, etc., can solve problems such as unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Example 1: Antibodies are internalized
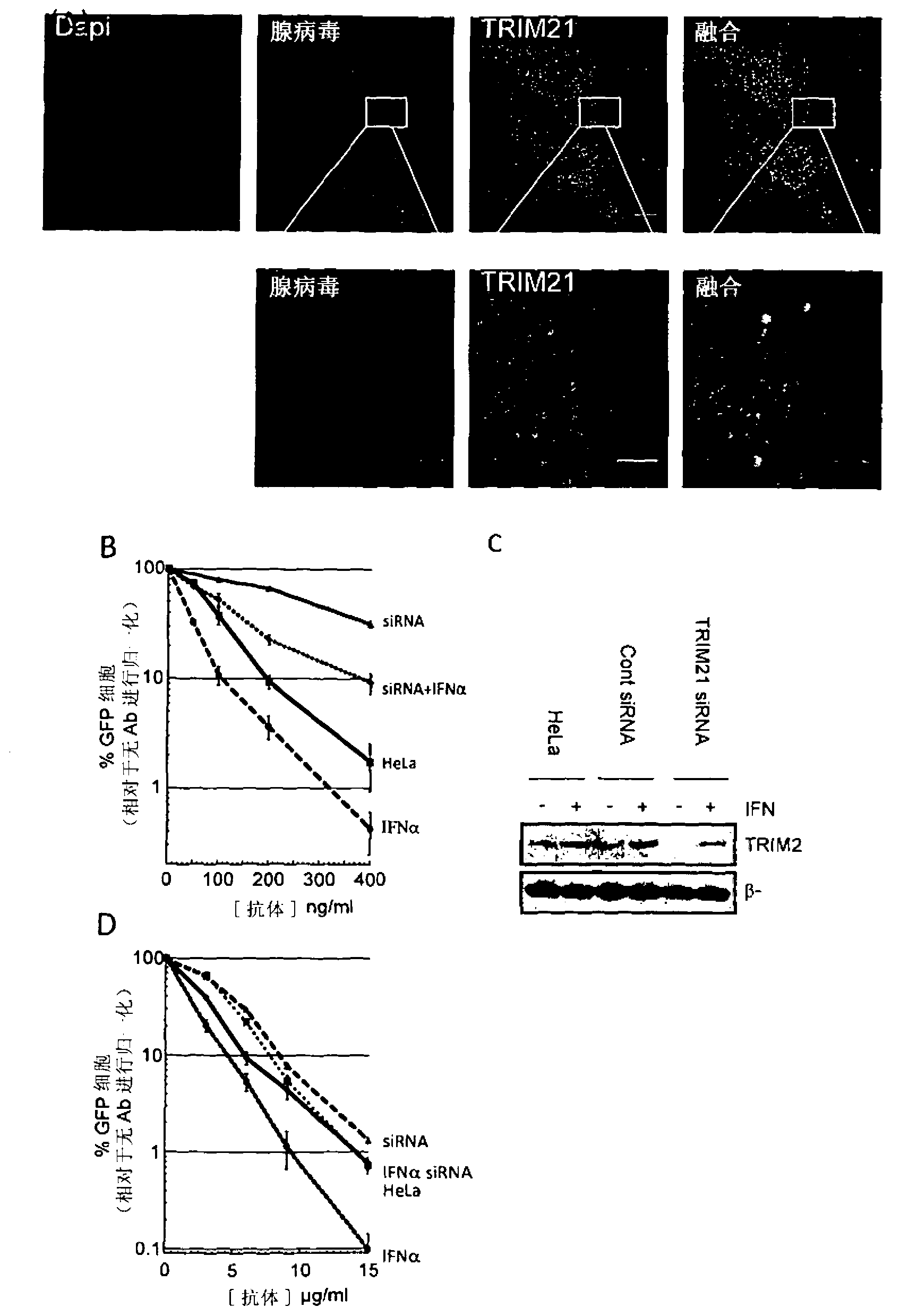
[0185] Antibodies are thought to not normally enter the cytosol during viral infection. To test this claim, we pre-incubated adenovirus (a human virus model that causes respiratory disease) with antibodies and added viral particles to cultured HeLa cells. Adenovirus was chosen because it is a non-enveloped virus whose capsid is naturally exposed to serum antibodies prior to cell infection. Thirty minutes after infection, cells were fixed, and a fluorescent anti-IgG antibody was added to detect antibody-coated viral particles. as in figure 1 As seen in A, antibody-coated virus particles successfully infect cells. Similar results were obtained using polyclonal anti-hexon antibodies and human serum IgG. Adenoviruses enter cells by binding to CAR receptors and are endocytosed. We found that the addition of antibodies did not prevent this process, and the antibodies remained attached to the virus after entry.
Embodiment 2
[0186] Example 2: TRIM21-mediated neutralization of intracellular virus
[0187] To understand whether cytosolic TRIM21 is accessible to antibody-coated viruses, we co-stained for TRIM21. Such as figure 1 In A, TRIM21 is efficiently recruited to antibody-coated virus particles.
[0188] We then examined the role of TRIM21 recruitment to viral particles by quantifying the level of adenovirus infection. We used viruses carrying the GFP gene so that the efficiency of infection could be determined by flow cytometry analysis. Standard virus neutralization assays were performed on HeLa cells pretreated with control siRNA, TRIM21 siRNA, interferon-α (IFNα), or IFNα and TRIM21 siRNA ( figure 1 B). To account for the cytotoxicity and variable cell death between these different conditions, we measured the reduction in infection due to the addition of antibodies. In the absence of antibodies, adenovirus infects -50% of cells. The percentage of infected cells decreased rapidly with ...
Embodiment 3
[0190] Example 3: Variation of Conditions
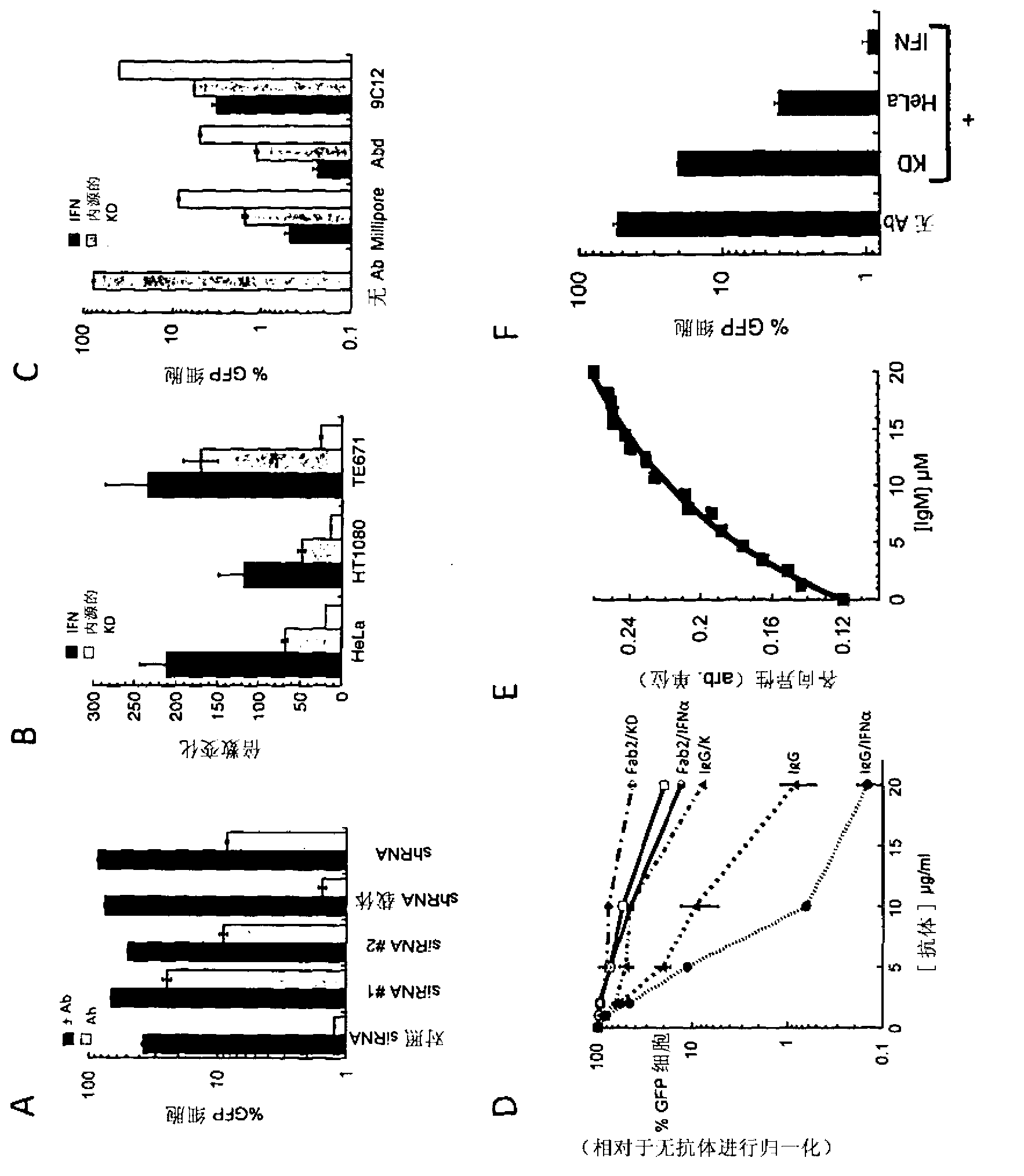
[0191] To confirm that TRIM21 / antibody intracellular neutralization is not adenovirus specific, we examined its effect on Coxsackievirus B3 infection. Coxsackievirus B3 is a picornavirus of the same genus as poliovirus and is the leading cause of aseptic meningitis. HeLa cells pretreated with the combination of TRIM21 siRNA and IFNαA as described above were infected with a replication competent strain carrying the GFP receptor gene. Infection time is limited to figure 1 D). Removal of TRIM21 in IFNα-treated cells restored the level of infection to that of untreated cells, demonstrating a TRIM21-dependent effect.
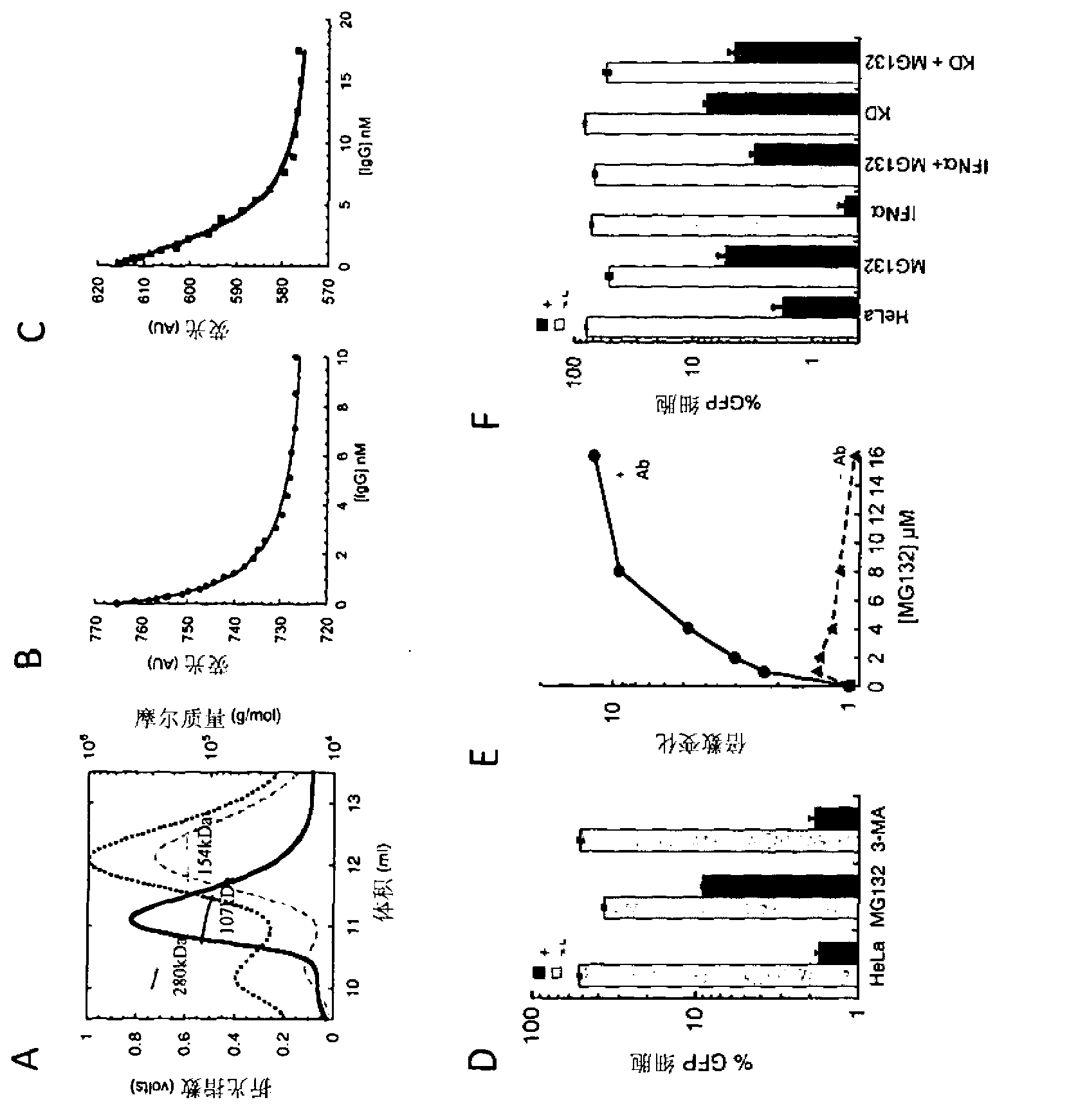
[0192] We confirmed the robustness of this phenotype by examining the effect of different siRNA sequences, cell types and antibody types. Such as figure 2 As seen in A, different TRIM21 siRNAs with different target sequences reversed antibody neutralization of adenovirus infection by knockdown of TRIM21 levels. Second...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com