Protein combined chip for Lyme disease immunoserology diagnosis and preparation method and application thereof
A protein and chip technology, applied in the field of biomedical detection, to achieve the effect of reducing non-specificity, avoiding false positives, simple and reliable detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The selection and surface chemical treatment of embodiment 1 carrier
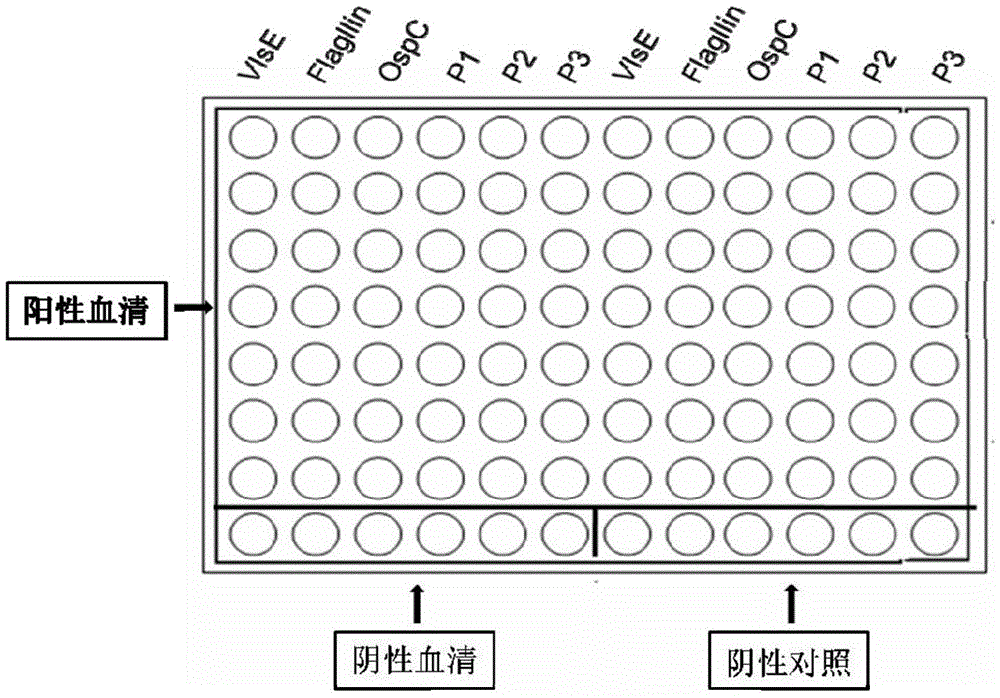
[0081] The present invention selects a gold foil chip from Interactiva Company (Ulm, Germany). Its substrate is a glass sheet covered with a layer of pure gold (purity 99.9%) with a thickness of 10nm. On the gold foil is a regionalized 50μm TEFLON Membrane array (96 holes*2, 8 rows*12 columns), the array aperture is 1.25mm, such as figure 1 shown.
[0082] Step 1. Cleaning of gold foil chips:
[0083] Prepare TL1 solution (H 2 O:H 2 o 2 :NH 3 ·H 2 O=5:1:1) into a stainless steel box, put the chip into the box, bathe in 82°C water for 6 minutes, rinse with deionized water 4-5 times, ethanol twice, each time for 3 minutes; air-dry with nitrogen, dry and store.
[0084] Step 2. Chemically modify the surface of the cleaned gold foil chip to obtain a solid phase carrier
[0085]Spot the modification solution, that is, a solution of dithiobis(succinimidyl undecanoate) (DSU) in dimethyl sulfoxide (D...
Embodiment 2
[0087] Embodiment 2 quality control experiment
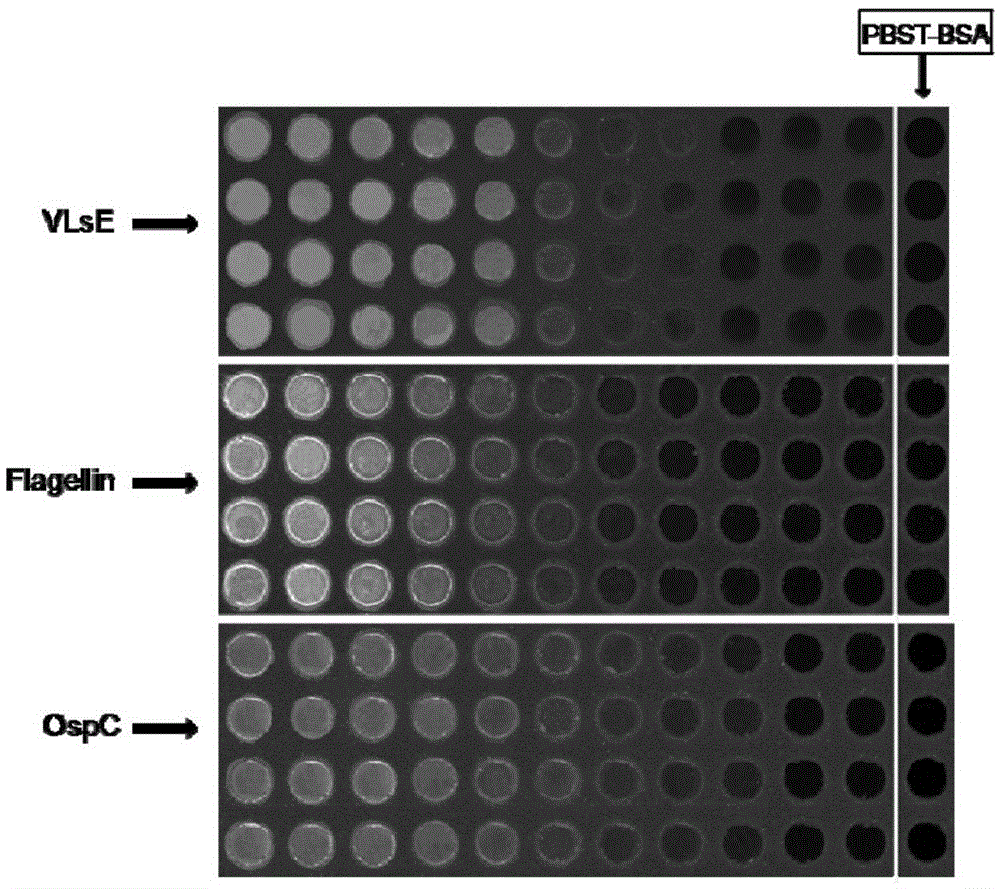
[0088] Preparation of incubation solution 1: Dissolve VlsE, Flagellin, and OspC antigens in PBST-BSA solution, respectively, and prepare concentration gradients of 200 μg / mL, 100 μg / mL, 50 μg / mL, 25 μg / mL, 12.5 μg / mL, 6.25 μg / mL mL, 3.13μg / mL, 1.56μg / mL, 0.78μg / mL, 0.39μg / mL, 0.19μg / mL antigen solution.
[0089] Prepare incubation solution 2: Dissolve rabbit anti-VlsEIgG antibody, rabbit anti-FlagellinIgG antibody, and rabbit anti-OspCIgG antibody in PBST-BSA solution, respectively, and prepare antibody concentration gradients of 200 μg / mL, 100 μg / mL, 50 μg / mL, and 25 μg / mL , 12.5 μg / mL, 6.25 μg / mL, 3.13 μg / mL, 1.56 μg / mL, 0.78 μg / mL, 0.39 μg / mL, 0.19 μg / mL solution.
[0090] Preparation of incubation solution 3: Dissolve Cy3-labeled goat anti-rabbit IgG antibody in PBST-BSA solution to prepare a solution with a concentration of fluorescent secondary antibody of 2.5 μg / mL.
[0091] Prepare incubation solution 4: Dissolve equal...
Embodiment 3
[0103] Example 3 Detection of Serum Samples from Lyme Disease Patients
[0104] Sera from 56 clinically diagnosed patients with Lyme disease were selected and divided into two groups, with 28 serum samples from clinically diagnosed patients with Lyme disease in each group, which were detected by the combined protein chip and detection method of the present invention.
[0105]In the first group, the sera of 28 patients with clinically confirmed Lyme disease were spotted on 168 wells of the chip containing VlsE, Flagellin, OspC recombinant antigen, and three VlsEIR6 polypeptide probes at an incubation concentration of 50 μg / mL. , the other 12 wells were sampled with serum from healthy people who were clinically confirmed not infected with Borrelia burgdorferi, and the other 12 wells were sampled with PBST-BSA solution, incubated at room temperature for 1 hour, washed with PBST 3 times after removal, Blow dry with nitrogen gas for 2 minutes each time. In the second group, the se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com