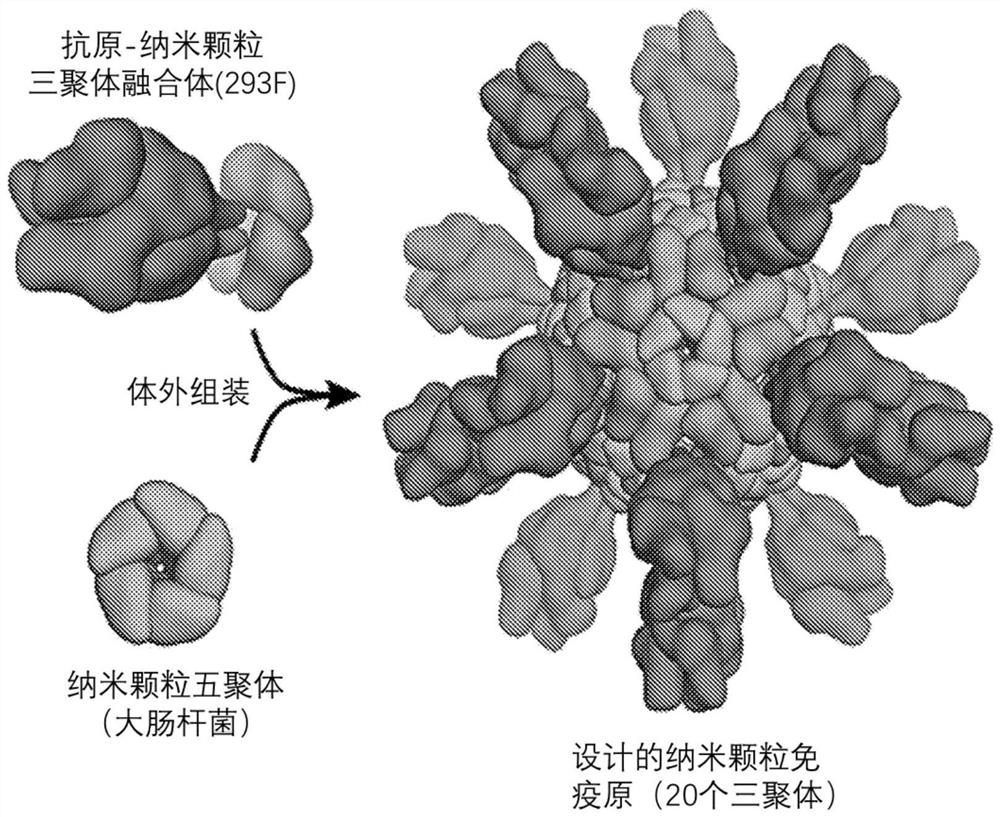
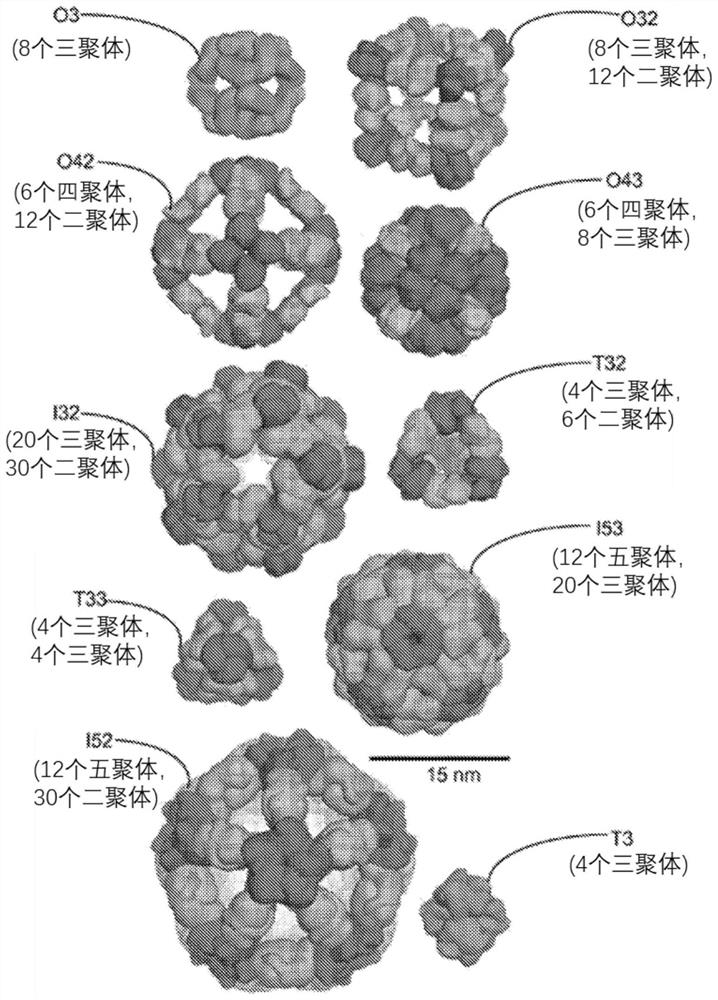
Self-asssembling nanostructure vaccines
A nanostructure and vaccine technology, applied in nanotechnology, nanotechnology, nanomedicine, etc., can solve problems such as weak immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0232] 9.1. Example 1: Respiratory syncytial virus (RSV)
[0233] 9.1.1. Sequence
[0234] In an embodiment of the present disclosure, the RSV F protein exists as a fusion protein with the first polypeptide and a linker is used, and the F protein-linker sequence may include the following:
[0235] >DS-Cav1-foldon (SEQ ID NO:90)
[0236](MELLILKANAITTILTAVTFCFASG)QNITEEFYQSTCSAVSKGYLSALRTGWYTSVITIELSNIKENKCNGTDAKVKLIKQELDKYKNAVTELQLLMQSTPATNNRARRELPRFMNYTLNNAKKTNVTLSKKRKRRFLGFLLGVGSAIASGVAVCKVLHLEGEVNKIKSALLSTNKAVVSLSNGVSVLTFKVLDLKNYIDKQLLPILNKQSCSISNIETVIEFQQKNNRLLEITREFSVNAGVTTPVSTYMLTNSELLSLINDMPITNDQKKLMSNNVQIVRQQSYSIMCIIKEEVLAYVVQLPLYGVIDTPCWKLHTSPLCTTNTKEGSNICLTRTDRGWYCDNAGSVSFFPQAETCKVQSNRVFCDTMNSLTLPSEVNLCNVDIFNPKYDCKIMTSKTDVSSSVITSLGAIVSCYGKTKCTASNKNRGIIKTFSNGCDYVSNKGVDTVSVGNTLYYVNKQEGKSLYVKGEPIINFYDPLVFPSDEFDASISQVNEKINQSLAFIR(KSDELL) GYIPEAPRDGQAYVRKDGEWVLLSTFL
[0237] In various further embodiments, the first polypeptide comprises or consists of a first polypept...
Embodiment 2
[0326] 9.2. Example 2: Cytomegalovirus (CMV)
[0327]Protein-based vaccines for CMV are described, eg, in US Patent Publication Nos. US 2016 / 0159864A1 and US 2017 / 0369532A1; International Patent Publication No. WO 2016 / 092460A3; and Kirchmeier et al. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B antigen induces antibodies with potent and broadly neutralizing activity. Clin Vaccine Immunol. 2014;21(2):174–80. The homotrimeric complex of gB, the trimeric gH / gL / gO complex, or the pentameric gH / gL / pUL128 / pUL130 / pUL131A complex are considered three major targets for CMV vaccination.
[0328] The first of these targets, gB, forms a trimeric structure comprising several hydrophobic surfaces. The C-terminus of the extracellular domain of gB is proximal to the transmembrane region and is located near the 3-fold axis of the molecule. See Chandramouli et al. Structure of HCMV glycoprotein B in the postfusion conformation bound to aneutralizing human ...
Embodiment 3
[0331] 9.3. Example 3: Epstein-Barr Virus (EBV)
[0332] Epstein-Barr virus (EBV) represents a major global health concern. Although it is associated with infectious mononucleosis and an estimated 200,000 cases of cancer each year worldwide, there is no vaccine available. The main target of immunity is EBV glycoprotein 350 / 220 (gp350), which mediates attachment to B cells via complement receptor 2 (CR2 / CD21). See Kanekiyo et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell 162(5):1090-1100 (2015). The extracellular domain of gp350 or the D of gp350 123 Fragments are expressed as gene fusions with nanostructured polypeptides, either as N-terminal or C-terminal fusions. The resulting gene fusions are expressed, assembled, and formulated into nanostructure-based vaccines. Antigenicity was determined using monoclonal antibodies 72A1 and 2L10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com