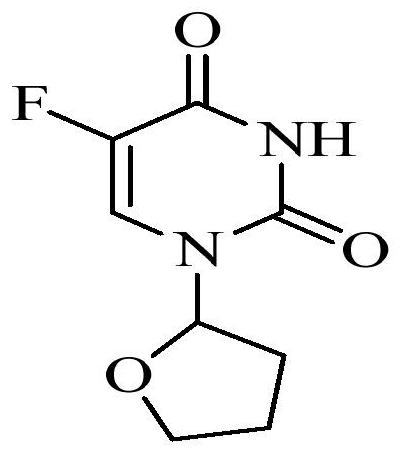
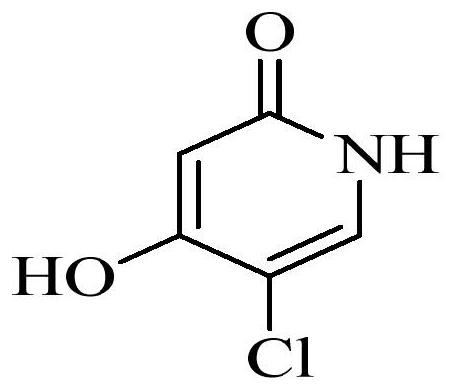
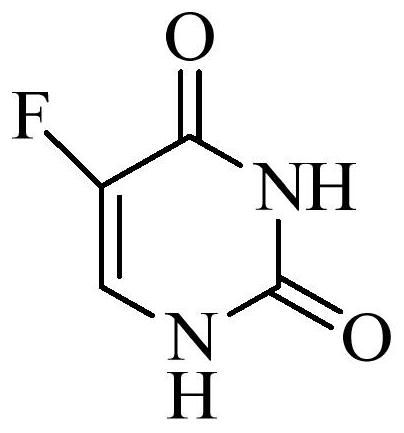
Method for determining concentration of tegafur, gimeracil and 5-fluorouracil in plasma of tumor patient
A technology of gimeracil and fluorouracil, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of low signal-to-noise ratio, long chromatographic running time, and insufficient sensitivity of 5-fluorouracil to fully describe the pharmacokinetic characteristics, etc., to achieve sensitive High, fast detection speed, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0037] pre-processing
[0038] The amount of plasma used in the present invention is only 50.0 μL, and the extraction method in the present invention is the protein precipitation method, which has a high recovery rate for polar substances, and has the advantages of simple operation, short extraction time, high-quality and high-quality suitable for clinical research. Quantitative sample pretreatment.
[0039] like Figure 1 to Figure 6 , the specific preprocessing steps are as follows:
[0040] 1. Add 50.0 μL plasma sample to the 96-well plate;
[0041] 2. Add 25.0 μL internal standard solution (FT- 13 C, 15 N 2 , CDHP- 13 C 3 , 5-FU- 13 C, 15 N 2 The concentration is 1500 / 300 / 150ng / mL respectively);
[0042] 3. Add 200 μL of acetonitrile; vortex for 10 minutes, centrifuge (4°C) for 10 minutes (4500 rpm);
[0043] 4. Take 100 μL of the supernatant and dry it in a nitrogen stream to concentrate;
[0044] 5. The residue was dissolved in 120 μL of reconstitution solvent...
Embodiment 1
[0054] Abbreviation
[0055]
[0056] 1 material
[0057] 1.1 Instrument
[0058] Chromatograph: LC-30AD fast liquid chromatography system, Shimadzu Corporation, Japan.
[0059] Mass spectrometer: 6500 + A type triple quadrupole tandem mass spectrometer equipped with an electrospray ionization source (Turbo Ion Spray) from Sciex, Canada.
[0060] Software used for data processing: Analyst (version 1.6.3), Sciex, Canada.
[0061] Centrifuge: HerμLe Z2326K desktop centrifuge, Germany Hermer Company.
[0062] Analytical balance: CPA225D analytical balance, Beijing Sartorius Instrument Co., Ltd.
[0063] 1.2 Reference substances and reagents
[0064] Tegafur (content 99.71%), gimeracil (purity 99.55%), and 5-fluorouracil (purity 99.85%) were purchased from Dalian Meilun Biotechnology Co., Ltd. 13 C 15 N 2 - Tegafur, 13 C 3 - Gimeracil, 13 C 15 N 2 - Fluorouracil was purchased from TRC. Methanol (HPLC grade) and acetonitrile (HPLC grade) were purchased from Sigma,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com