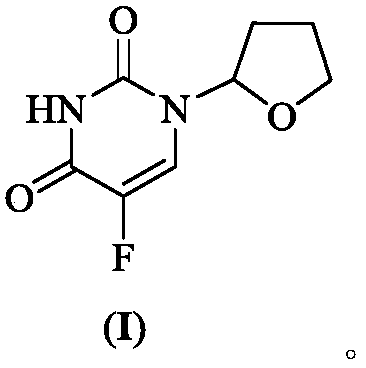
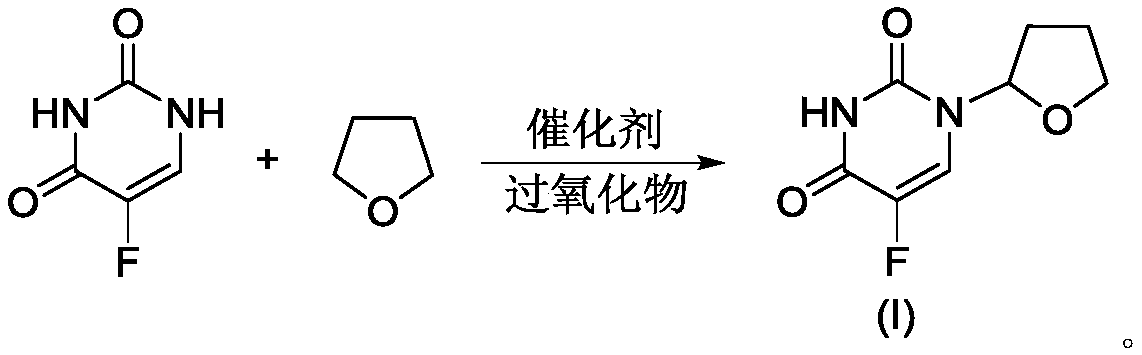
Preparation method of tegafur
A technology of tegafur and fluorouracil, which is applied in the field of medicinal chemistry, can solve the problems of many impurities and cumbersome operations, and achieve the effects of high reaction selectivity, efficient reaction process, and increased selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 5-fluorouracil (13.00g, 0.10mol), tetrahydrofuran (11.54g, 0.16mol), copper acetate (399.30mg, 2.0mmol), hydrogen peroxide solution (28.33g, 0.25mol, 30%), N,N- Add dimethylformamide (150mL) into the flask, start stirring, heat to 40°C, react for 5h, filter, pour the filtrate into a mixture of ice water (500mL) and ethyl acetate (300mL), stir for 15min, and divide liquid, and the organic phase was washed with purified water (100mL×3) and saturated brine (100mL) successively, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain crude tegafur, which was recrystallized from anhydrous methanol to obtain Tegafur refined product, yield 92.35%, detected by HPLC, wherein t R =13.926min is tegafur with a purity of 99.952% and a maximum of 0.016% impurity.
Embodiment 2
[0038] 5-fluorouracil (13.00g, 0.10mol), tetrahydrofuran (7.21g, 0.10mol), copper acetate (399.30mg, 2.0mmol), hydrogen peroxide solution (28.33g, 0.25mol, 30%), N,N- Add dimethylformamide (150mL) into the flask, start stirring, heat to 40°C, react for 5h, filter, pour the filtrate into a mixture of ice water (500mL) and dichloromethane (300mL), stir for 15min and divide liquid, and the organic phase was washed with purified water (100mL×3) and saturated brine (100mL) successively, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain crude tegafur, which was recrystallized from anhydrous methanol to obtain Tegafur refined product, yield 90.16%, detected by HPLC, wherein t R =13.875min is tegafur, the purity is 99.894%, and the maximum is 0.019%.
Embodiment 3
[0040] 5-fluorouracil (13.00g, 0.10mol), tetrahydrofuran (14.42g, 0.20mol), copper acetate (399.30mg, 2.0mmol), hydrogen peroxide solution (28.33g, 0.25mol, 30%), N,N- Add dimethylformamide (150mL) into the flask, start stirring, heat to 40°C, react for 5h, filter, pour the filtrate into a mixture of ice water (500mL) and ethyl acetate (300mL), stir for 15min, and divide liquid, and the organic phase was washed with purified water (100mL×3) and saturated brine (100mL) successively, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain crude tegafur, which was recrystallized from anhydrous methanol to obtain Tegafur refined product, yield 91.38%, detected by HPLC, wherein t R =13.863min is tegafur, with a purity of 99.927% and a maximum of 0.016%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com