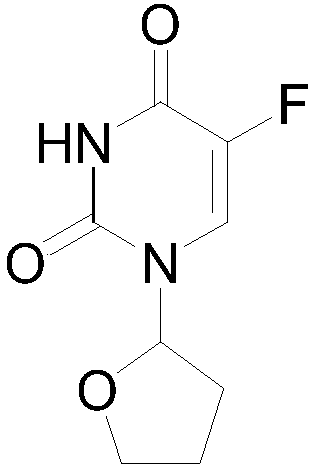
A kind of preparation method of tegafur
A technology of tegafur and fluorouracil, which is applied in the field of drug synthesis, can solve problems such as harsh reaction conditions and complex protective groups, and achieve the effects of mild reaction conditions, high yield, and simple product purification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 100 grams (0.769 mol) of 5-fluoro-uracil, 83.5 grams (1.192 mol) of 2,3-dihydrofuran, 800 milliliters of acetonitrile, and 8.7 grams of triethylamine in the flask, cool down to 15°C, and add 148.5 grams of (1.586mol) dimethylchlorosilane, react at 16°C for 2-3 hours, after the reaction is complete, pour the reaction solution into 1000 ml of ice water, adjust the pH of the solution to 9 with sodium hydroxide, and stir at room temperature for 30 minutes , extracted twice with 300 milliliters of petroleum ether, discarded the organic phase, adjusted the pH value of the aqueous phase to 4.5 with hydrochloric acid, extracted three times with 400 milliliters of dichloromethane, dried the organic phase with anhydrous magnesium sulfate, and concentrated to dryness under reduced pressure to obtain shallow yellow solid.
[0021] The obtained light yellow solid was added to a flask, 860 ml of acetone was added, heated to dissolve at 50°C, crystallized at -20°C for 4 hours, and...
Embodiment 2
[0023] Add 100 grams (0.769 mol) of 5-fluoro-uracil, 96 grams (1.371 mol) of 2,3-dihydrofuran, 800 milliliters of acetonitrile, and 8.7 grams of triethylamine into the flask, cool down to 15°C, and add 200 grams of (2.137mol) dimethylchlorosilane, react at 18°C for 2-3 hours, after the reaction is complete, pour the reaction solution into 1000 ml of ice water, adjust the pH of the solution to 9 with sodium hydroxide, and stir at room temperature for 30 minutes , extracted three times with 300 milliliters of petroleum ether, discarded the organic phase, adjusted the pH value of the aqueous phase to 4.4 with hydrochloric acid, extracted three times with 400 milliliters of dichloromethane, dried the organic phase with anhydrous magnesium sulfate, and concentrated to dryness under reduced pressure to obtain light yellow solid
[0024] The obtained light yellow solid was added to a flask, 860 ml of acetone was added, heated and dissolved at 50°C, crystallized at 0°C for 8 hours, ...
Embodiment 3
[0026] Add 100 grams (0.769 mol) of 5-fluoro-uracil, 150 grams (2.14 mol) of 2,3-dihydrofuran, 800 milliliters of acetonitrile, and 8.7 grams of triethylamine into the flask, cool down to 15°C, and add 108.5 grams of (1.16mol) dimethylchlorosilane, react at 16°C for 2-3 hours, after the reaction is complete, pour the reaction solution into 1000 ml of ice water, adjust the pH of the solution to 9 with sodium hydroxide, and stir at room temperature for 30 minutes , extracted twice with 300 milliliters of petroleum ether, discarded the organic phase, adjusted the pH value of the aqueous phase to 4.5 with hydrochloric acid, extracted three times with 400 milliliters of dichloromethane, dried the organic phase with anhydrous magnesium sulfate, and concentrated to dryness under reduced pressure to obtain shallow yellow solid.
[0027] The obtained light yellow solid was added to a flask, 860 ml of acetone was added, heated to dissolve at 50°C, crystallized at -10°C for 6 hours, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com