Carmustine sustained-release implant for treating solid tumor and preparation method thereof
A slow-release implant, carmustine technology, applied in the field of medicine, can solve the problems of short release period and instability, and achieve the effects of enhanced sensitivity, enhanced effect, and enhanced anti-cancer effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Embodiment 1, compared the difference of the selected adjuvant of the present invention and polyphenylene
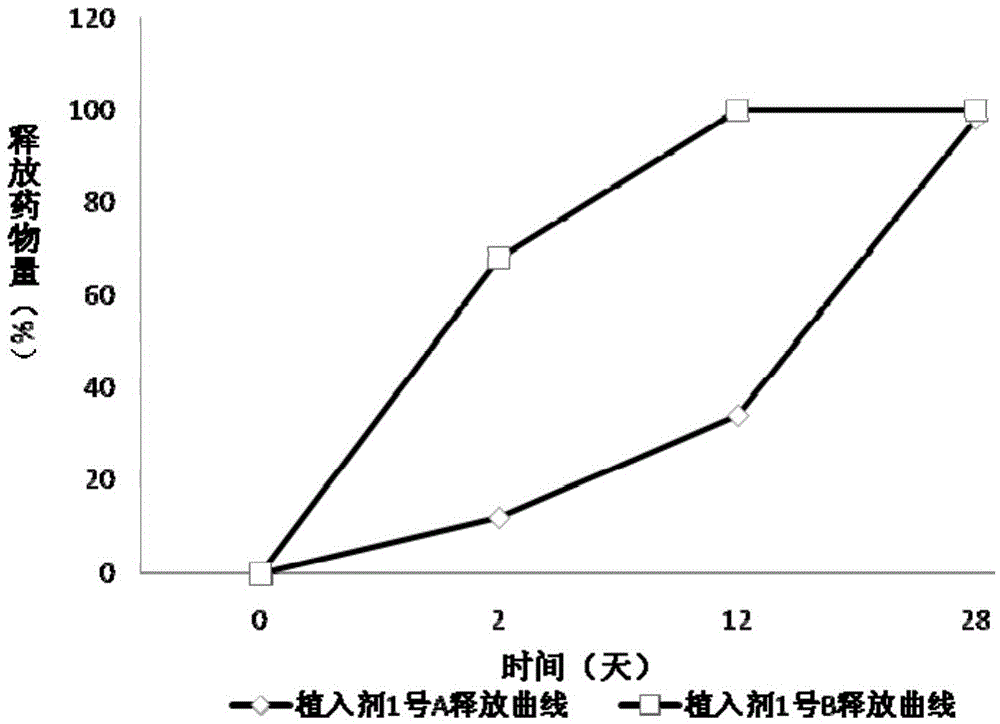
[0101] Mix 9.0g PLGA (the mass ratio of lactide to glycolide is 50:50, the viscosity range is 0.30dl / g) and 9.0g polyphenylene propane (p-CPP): sebacic acid ( SA) copolymer, 80:20) into containers "A" and "B", respectively, add 100ml of dichloromethane to dissolve and mix well, then add 1.0g carmustine, re-shake well, pour into the tray to heat and dry Remove organic solvents. Freeze and pulverize the dried solid composition, put the powder into a tablet machine to form after the organic residue is qualified, make sustained-release tablets at room temperature, sterilize with cobalt 60 after sub-packaging, and the obtained product is "implantation agent" No. 1", is a slow-release implant, with a diameter of 1.4 cm and a thickness of 1.0 mm, wherein,
[0102] Implant No. 1 A is: 10% carmustine, the excipient is PLGA (the ratio of lactide to glycolide is 50:50, the...
Embodiment 2
[0107] Embodiment 2, the impact of different molecular weight copolymers on BCNU release
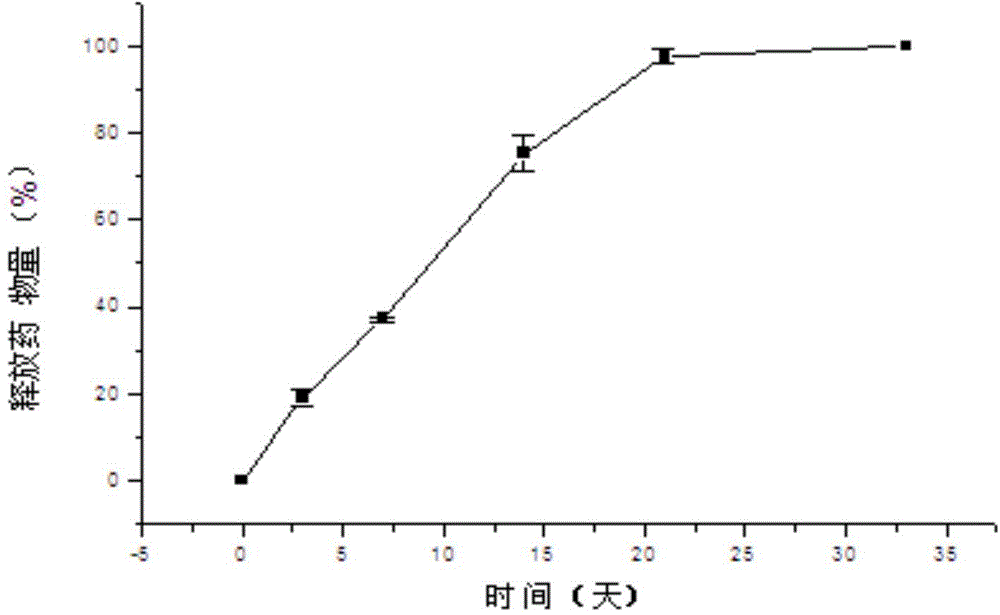
[0108] The sustained-release implant tablet was prepared according to the preparation process of Example 1, except that the PLGA used was a copolymer of lactide and glycolide with a mass ratio of 50:50, and the weight average molecular weight was 15K, 45K and 75K respectively, and the resulting product was Implant No. 2 (A, B, C) has a diameter of 1.35-1.45 cm and a thickness of 0.8-1.2 mm, all containing 10% carmustine. The drug release results of implant No. 2 in pure water are shown in Table 2:
[0109] Table 2
[0110]
[0111] The above results show that, in the case of the same drug loading (10%), the release characteristics of carmustine implants made of lactide-glycolide copolymers are related to the molecular weight of the carrier, and the implants with a weight average molecular weight of 15K The implant (implant No. 2 A) releases too fast, and releases 76.98% on ...
Embodiment 3
[0112] Embodiment 3, compare the impact of different molecular weight copolymers on BCNU release
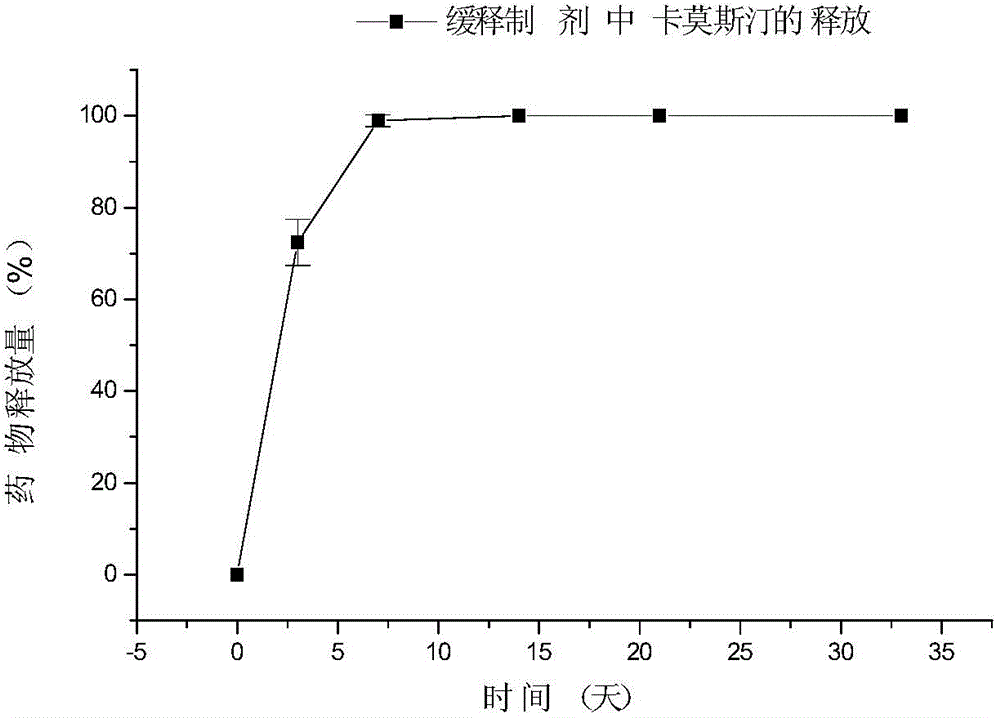
[0113] Sustained-release implant tablets were prepared according to the preparation process of Example 1, except that PLGA was a lactide-glycolide copolymer with a mass ratio of 50:50, and the weight-average molecular weights were 25K, 40K and 65K respectively, and the resulting product The No. 3 implant (A, B, C) has a diameter of 1.35-1.45 centimeters and a thickness of 0.8-1.2 millimeters, all containing 10% carmustine. Its release time in pure water see
[0114] table 3:
[0115] table 3
[0116]
2 days release percentage (%)
12-day release percentage (%)
28-day release percentage (%)
Implant No. 3 A
9.20
58.98
100
Implant No. 3 B
9.48
46.1
94.31
Implant No. 3 C
12.61
40.92
84.52
[0117] The above results show that, under the same drug loading (10%), the release characteristics of carmu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com