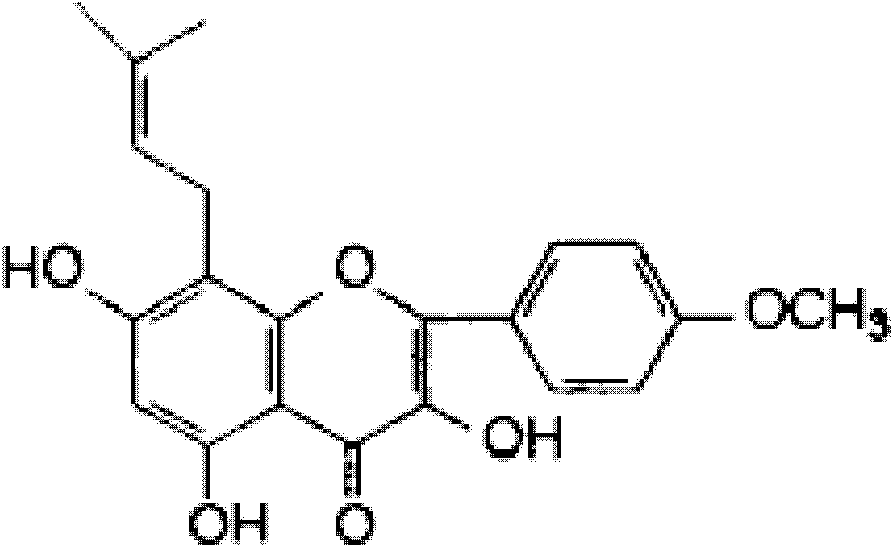
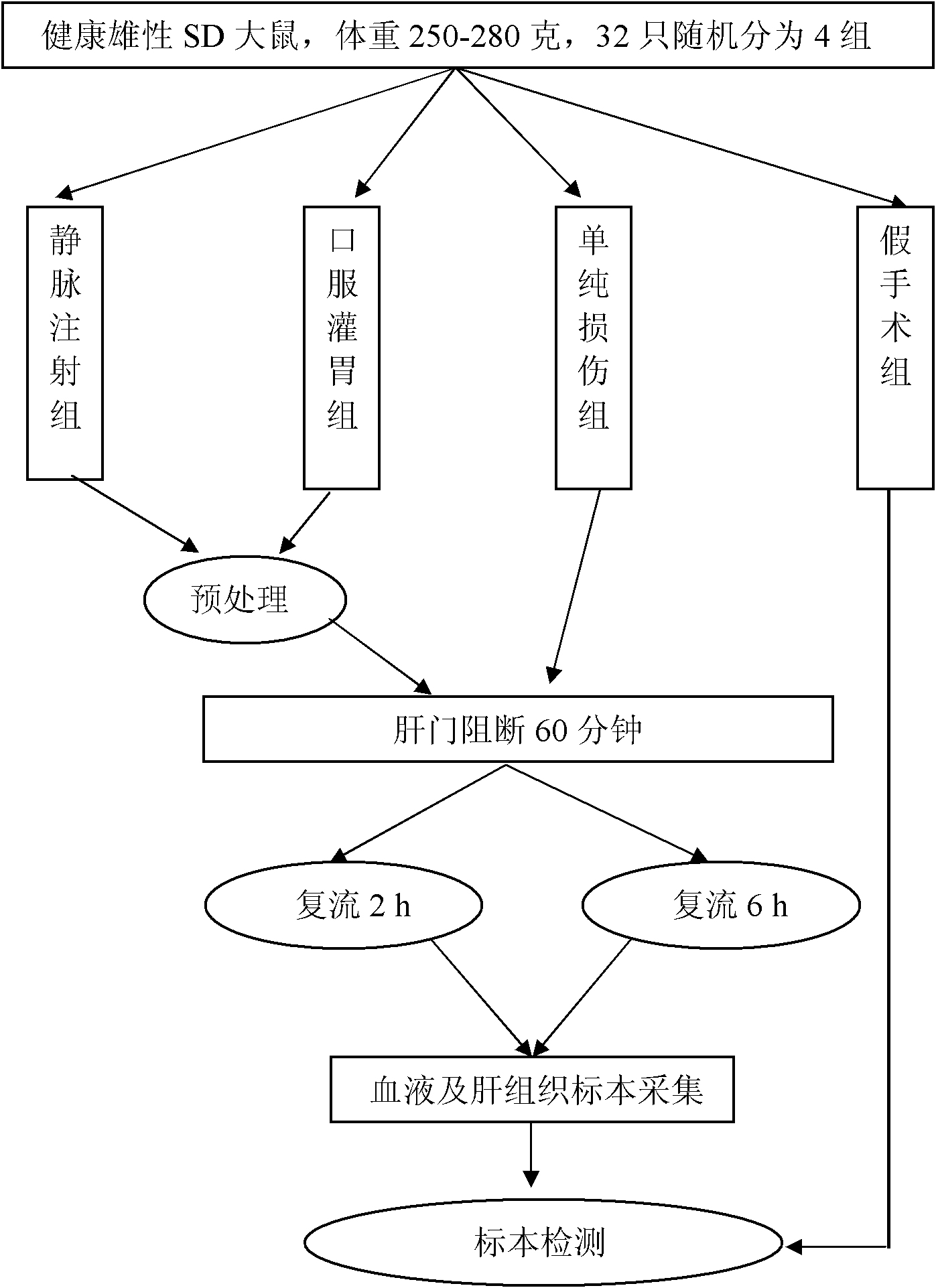
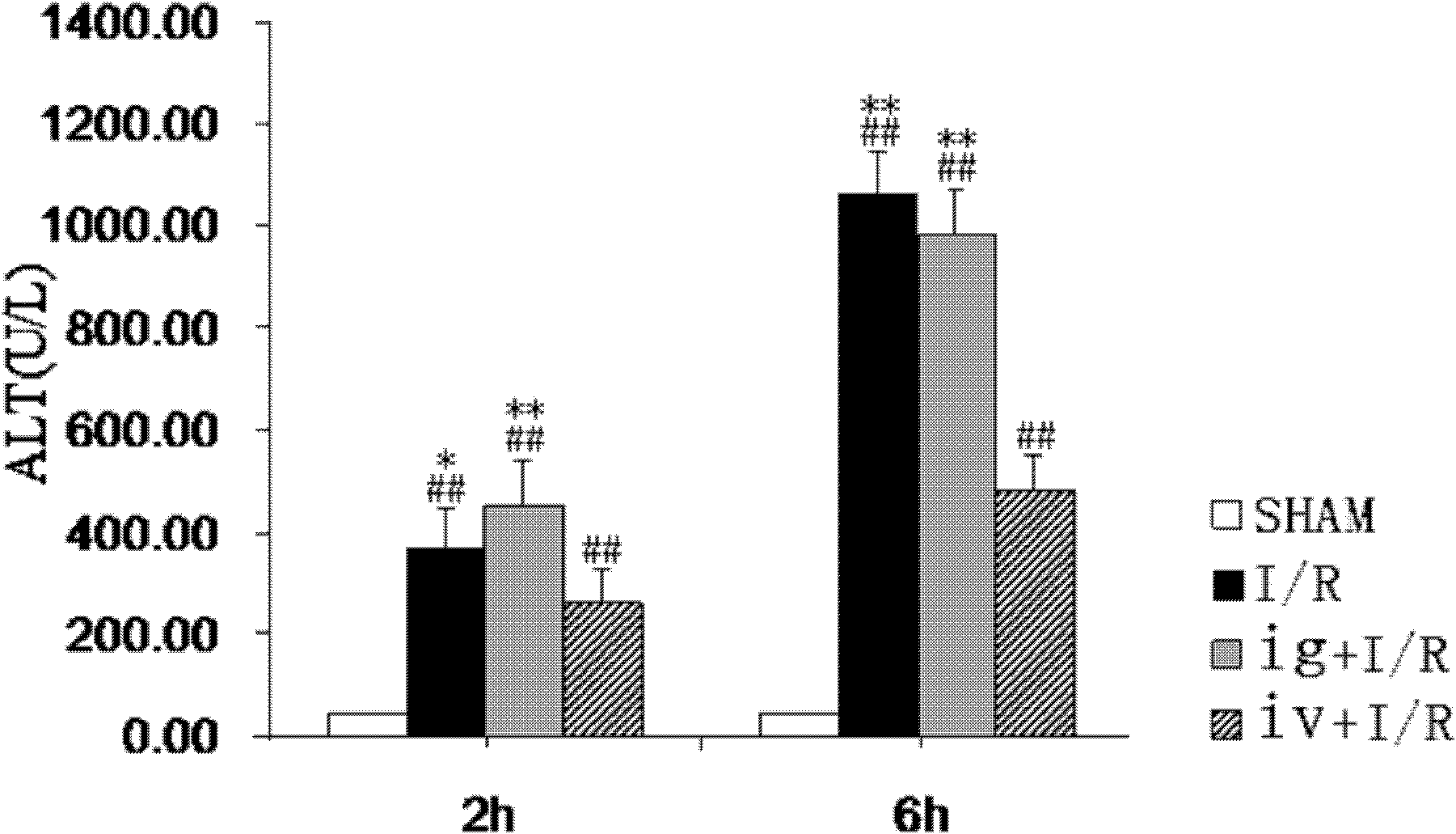
Icaritin liposome and preparation method thereof
A technology of icariin and liposome preparation, which is applied in the field of medicine, can solve the problems of poor therapeutic effect, stability of curative effect, lower therapeutic index, and poor curative effect, so as to reduce the dosage of drugs and toxic and side effects, and repair liver acute disease. Damage and toxicity reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of icariin liposome injection
[0033] Step 1: Weigh 1 g of epimeditin, 20 g of soybean lecithin and 2.5 g of cholesterol and put them in a round bottom flask;
[0034] Step 2: Add an appropriate amount of a mixed solution consisting of 8ml of ethanol and 4ml of chloroform to the above mixture to completely dissolve into a clear solution, and dry under reduced pressure at 65°C for about 4 hours by rotary thin film evaporation to completely evaporate the organic solvent;
[0035] Step 3: Add 800ml of water for injection, and continue to rotate under normal pressure until the film is completely hydrated;
[0036] Step 4: emulsify with a shear emulsifier for 5 minutes (rotating speed 2000 rpm) to obtain colostrum, homogenize the colostrum with a micro-jet high-pressure homogenizer (pressure 9000 psi) three times, and dilute the volume to 1000 ml with water for injection;
[0037] Step 5: adjust the pH to 5.0 with potassium dihydrogen phosphat...
Embodiment 2
[0038] Embodiment 2: the preparation of icariin liposome injection
[0039] Step 1: Weigh 1g of epimeditin, 30g of egg yolk lecithin, 30g of cholesterol and 5g of polysorbate 80 and place in a round bottom flask;
[0040] Step 2: Add 30ml of chloroform to the above mixture to completely dissolve into a clear solution, and dry under reduced pressure at 70°C for about 2 hours by rotary thin film evaporation to completely evaporate the organic solvent;
[0041] Step 3: Add 600ml of water for injection, and continue to rotate under normal pressure until the film is completely hydrated;
[0042] Step 4: emulsify with a shear emulsifier for 3 minutes (3000 rpm) to obtain colostrum, homogenize the colostrum with a micro-fluid high-pressure homogenizer (pressure 8000 psi) three times, and dilute the volume to 1000 ml with water for injection;
[0043] Step 5: adjust the pH to 6.0 with sodium dihydrogen phosphate buffer solution, pack in ampoules (or vials), and sterilize to obtain ic...
Embodiment 3
[0044] Embodiment 3: the preparation of icariin liposome injection
[0045] Step 1: Weigh 4g of epimeditin and 10g of soybean lecithin into a round bottom flask;
[0046] Step 2: Add 15ml of chloroform to the above mixture to completely dissolve into a clear solution, and dry under reduced pressure at 75°C for about 3 hours by rotary thin film evaporation to completely evaporate the organic solvent;
[0047] Step 3: Add 200ml of water for injection, and continue to rotate under normal pressure until the film is completely hydrated;
[0048] Step 4: Use a shear emulsifier to emulsify for 4 minutes (1000 rpm) to obtain colostrum, sonicate the colostrum with an ultrasonic instrument for 10 minutes, and dilute the volume to 1000ml with water for injection;
[0049] Step 5: adjust the pH to 4.5 with glacial acetic acid, pack in ampoules (or vials), and sterilize to obtain icariin liposomes for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com