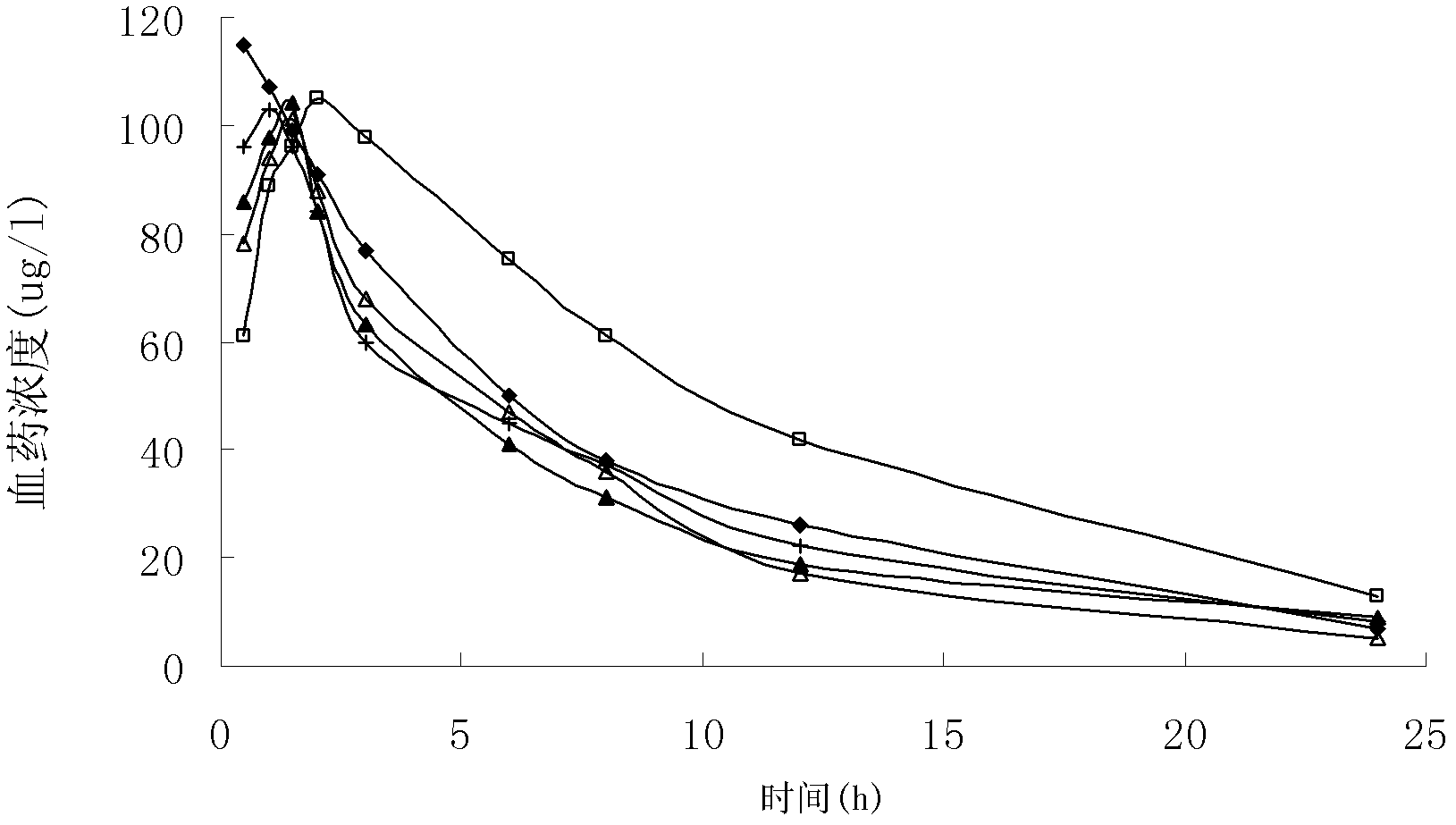
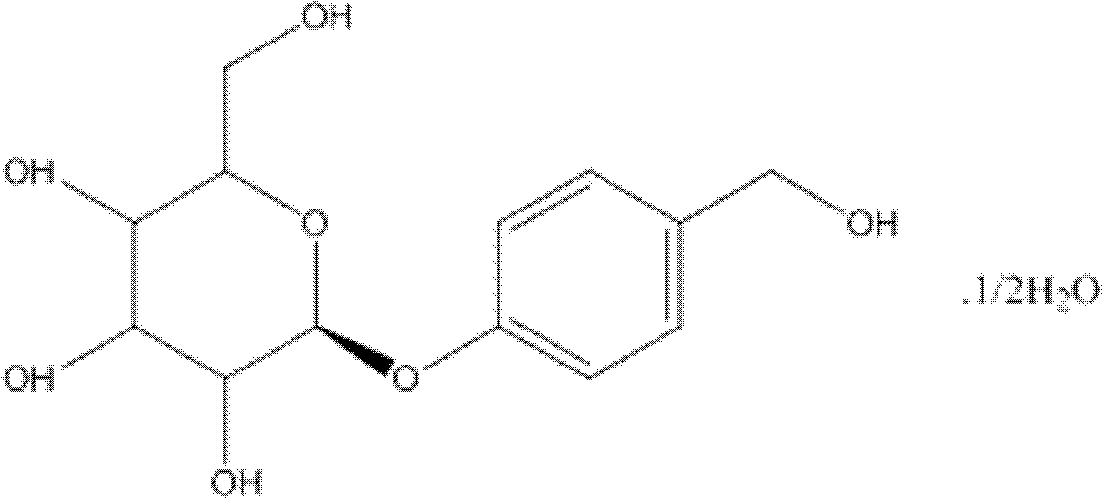
Gastrodin multiphase liposome injection
A multiphase liposome and gastrodin technology, applied in the field of medicine, can solve the problems of incomplete encapsulation of gastrodin and unsatisfactory compatibility, so as to improve bioavailability, improve product quality of preparations, and good encapsulation rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Preparation of Gastrodin Multiphase Liposome Injection
[0060] The components and weights of the raw and auxiliary materials used are as follows:
[0061]
[0062] The gastrodin heterogeneous liposome injection was prepared by the following preparation process:
[0063] (1) 50g Tween 80, 50g polyoxyethylene 40 hydrogenated castor oil, 200g cholesterol and 200g soybean phosphatidylinositol were dissolved in 500ml pH value of phosphate buffered saline solution of 6.8, stirred and ultrasonically pulverized twice, each 10 minutes each time, then add 60g trehalose and mix evenly, freeze-dry under vacuum condition, make the heterogeneous liposome of unencapsulated medicine;
[0064] (2) Dissolve 100g gastrodin in 200ml of phosphate buffer solution with a pH value of 6.8, dissolve it, and process it twice by ultrasonication for 10 minutes each time;
[0065] (3) Add gastrodin buffered saline and EDTA-2Na to the heterogeneous liposome of unencapsulated medicin...
Embodiment 2
[0067] Example 2 Preparation of Gastrodin Multiphase Liposome Injection
[0068] The components and weights of the raw and auxiliary materials used are as follows:
[0069]
[0070] The gastrodin heterogeneous liposome injection was prepared by the following preparation process:
[0071] (1) 70g Tween 80, 70g polyoxyethylene 40 hydrogenated castor oil, 300g cholesterol and 300g soybean phosphatidylinositol were dissolved in 1000ml pH value of phosphate buffered saline solution of 6.8, stirred, ultrasonic pulverization was processed twice, each 10 minutes each time, then add 40g trehalose and mix evenly, freeze-dry under vacuum condition, make the heterogeneous liposome of unencapsulated medicine;
[0072] (2) 200g gastrodin was dissolved in 400ml of phosphate buffered saline solution with a pH value of 6.8, dissolved, and subjected to ultrasonic treatment twice, each time for 10 minutes;
[0073] (3) Add gastrodin buffered saline and EDTA-2Na to the heterogeneous liposo...
Embodiment 3
[0075] Example 3 Preparation of Gastrodin Multiphase Liposome Injection
[0076] The components and weights of the raw and auxiliary materials used are as follows:
[0077]
[0078]
[0079] The gastrodin heterogeneous liposome injection was prepared by the following preparation process:
[0080] (1) 100g Tween 80, 100g polyoxyethylene 40 hydrogenated castor oil, 200g cholesterol and 400g soybean phosphatidylinositol were dissolved in 2000ml pH value of phosphate buffered saline solution of 6.8, stirred, ultrasonic pulverized twice, each 10 minutes each time, then add 150g trehalose and mix evenly, freeze-dry under vacuum condition, make the heterogeneous liposome of unencapsulated medicine;
[0081] (2) 500g gastrodin was dissolved in 1000ml of phosphate buffer solution with a pH value of 6.8, dissolved, and subjected to ultrasonic treatment twice, each time for 10 minutes;
[0082] (3) Add gastrodin buffered saline and EDTA-2Na to the heterogeneous liposome of unen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com