Edaravone liposome injection and new application thereof
A technology of edaravone lipid and injection, applied in liposome injection of edaravone and its preparation, a new application field for the treatment of thrombophlebitis, which can solve the problem that it is difficult to achieve the therapeutic effect and the quality cannot be guaranteed to be stable and stable Sex can not meet the clinical requirements and other problems, to achieve the effect of promoting stability, improving stability and reducing leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
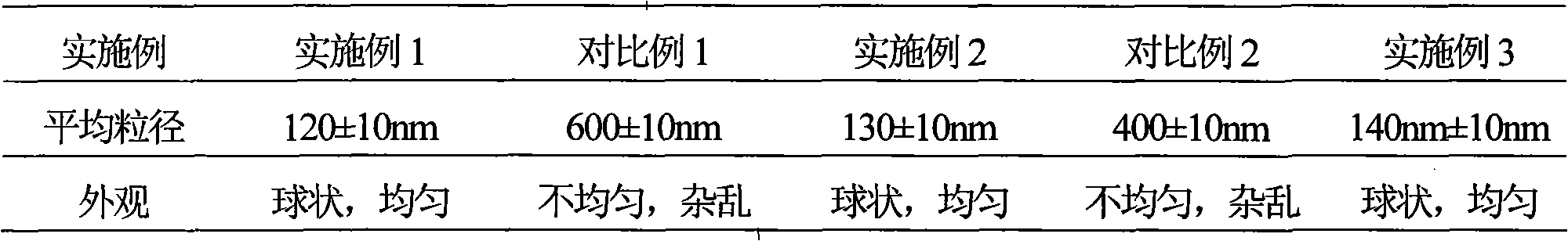
[0068] Example 1 Preparation of Edaravone Liposomes
[0069] Prescription (1000 sticks):
[0070] Edaravone 10g
[0071] Dilauroyl Phosphatidylcholine 200g
[0072] Cholesterol 5g
[0073] Polysorbate 80 10g
[0075] Acetic acid-sodium acetate buffer solution with a pH value of 5.0 500ml
[0076] Preparation Process
[0077] (1) Dissolve 200g of dilauroylphosphatidylcholine, 5g of cholesterol and 10g of polysorbate 80 in 1000ml of isopropanol, slowly inject 1000ml of 0.01mol / L ammonium sulfate solution under stirring, heat and stir to evaporate the isopropanol, Place in an ice bath and sonicate for 10 minutes to obtain blank liposomes;
[0078] (2) Blank liposome is placed in dialysis bag, seals, and dialysis bag is placed in 0.9% sodium chloride aqueous solution 1000ml and dialyzes 20 hours, removes the ammonium sulfate in liposome external phase;
[0079] (3) Dissolve 10g of Edaravone in 500ml of water, keep the dialyzed blank liposo...
Embodiment 2
[0088] Example 2 Preparation of Edaravone Liposomes
[0089] Prescription (1000 sticks):
[0090] Edaravone 15g
[0091] Hydrogenated Soy Lecithin 75g
[0092] Cholesterol 45g
[0093] Polysorbate 80 150g
[0094] Mannitol 225g
[0095] Boric acid-borax buffer solution with a pH value of 5.5 800ml
[0096] Preparation Process
[0097] (1) Dissolve 75g of hydrogenated soybean lecithin, 45g of cholesterol and 150g of polysorbate 80 in 1500ml of tert-butanol, slowly inject 1500ml of 0.1mol / L ammonium sulfate solution under stirring, heat and stir to remove tert-butanol, and place in an ice bath Ultrasound for 20 minutes to obtain blank liposomes;
[0098] (2) Place the blank liposome in the dialysis bag, seal it, place the dialysis bag in 1500ml of 10% mannitol aqueous solution and dialyze for 22 hours, remove the ammonium sulfate in the liposome external phase;
[0099] (3) 15g of Edaravone was dissolved in 800ml of water, and the dialyzed blank liposome was incubate...
Embodiment 3
[0112] Example 3 Preparation of Edaravone Liposomes
[0113] Prescription (1000 sticks):
[0114] Edaravone 30g
[0115] Egg Yolk Phosphatidylserine 300g
[0116] Cholesterol 150g
[0117] Polysorbate 80 120g
[0118] Glucose 240g
[0119] Phosphoric acid-disodium hydrogen phosphate buffer solution with a pH value of 6.0 1000ml
[0120] Preparation Process
[0121] (1) Dissolve 300g of egg yolk phosphatidylserine, 150g of cholesterol and 120g of polysorbate 80 in 2000ml of benzyl alcohol, slowly inject 2000ml of 0.15mol / L ammonium sulfate solution under stirring, heat and stir to remove benzyl alcohol, put it in an ice bath and sonicate 10min, get blank liposome;
[0122] (2) Blank liposome is placed in dialysis bag, seals, and dialysis bag is placed in 5% glucose aqueous solution 3200ml and dialyzes 20 hours, removes the ammonium sulfate in liposome external phase;
[0123] (3) Dissolve 30g of Edaravone in 1000ml of water, keep the dialyzed blank liposomes at 60°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com