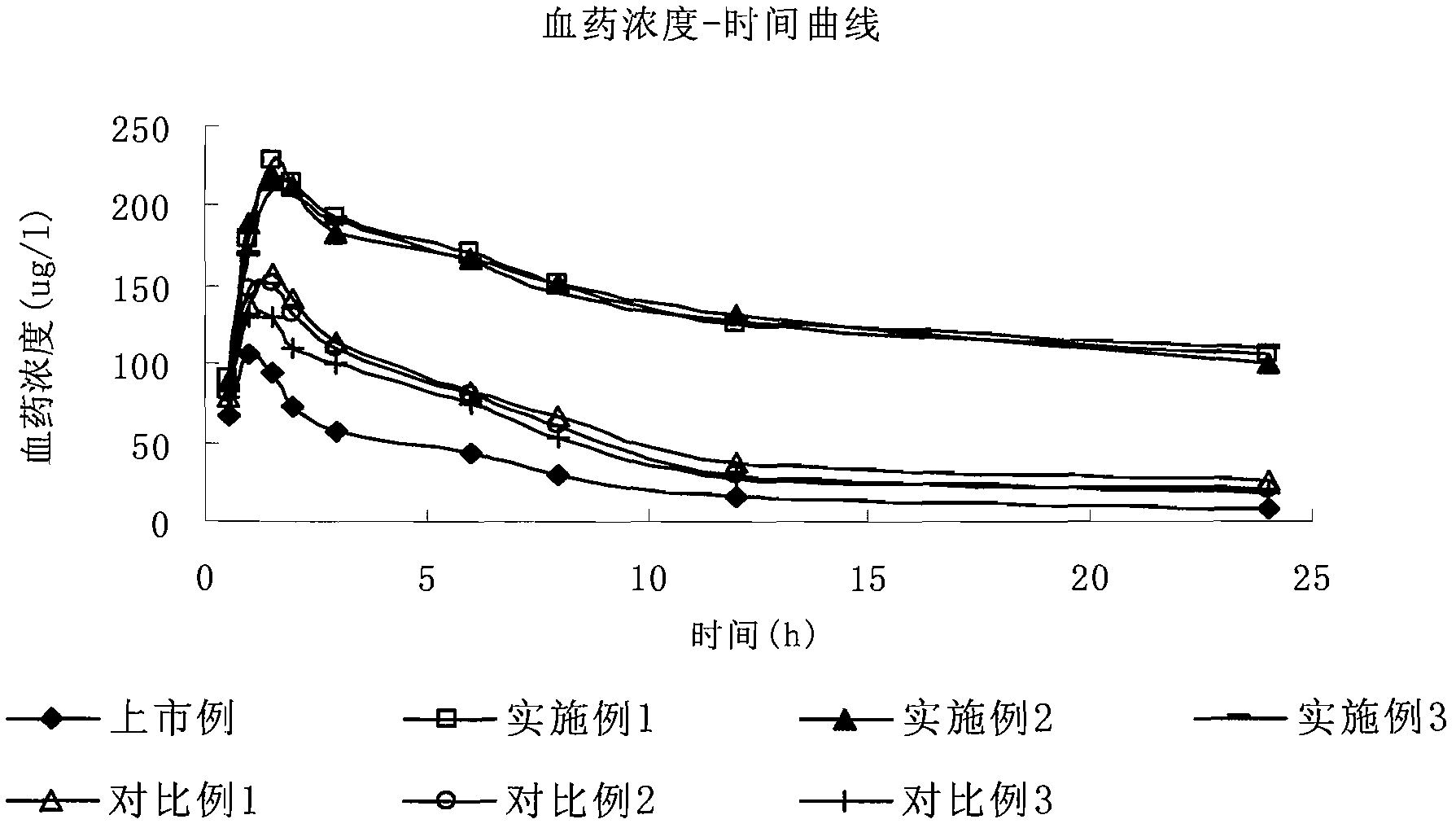
Ambroxol hydrochloride liposome injection
A technology for ambroxol hydrochloride and injection, which is applied in the fields of ambroxol hydrochloride injection and ambroxol hydrochloride liposome injection, can solve the problems of decreased drug quality, impurity, poor stability of physical and chemical properties, and inability of products to escape, Achieve the effect of improving stability, protecting form and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Preparation of Ambroxol Hydrochloride Liposomal Injection
[0072] Prescription: (1000 bottles)
[0073]
[0074] making process:
[0075] (1) Cholesterol 50g, soybean phosphatidylserine 80g, soybean sterol 30g and 20g Tween 80 are dissolved in the phosphate buffer solution that 2000mlpH is 7.2, make blank liposome;
[0076] (2) The blank liposomes prepared above were sterilized by flowing steam, and then ultrasonically treated twice, each time for 20 minutes;
[0077] (3) under aseptic conditions, add ambroxol hydrochloride 15g in the liposome of molten state, add 20g trehalose and 30gPVP under constantly stirring;
[0078] (4) Filter through a 0.45 μm microporous membrane, freeze quickly, then return to room temperature, and fill (2ml / bottle) to obtain ambroxol hydrochloride liposome injection.
Embodiment 2
[0079] Example 2 Preparation of Ambroxol Hydrochloride Liposomal Injection
[0080] Prescription: (1000 bottles)
[0081]
[0082] making process:
[0083] (1) Cholesterol 100g, soybean phosphatidylserine 160g, soybean sterol 60g and 40g Tween 80 are dissolved in 4000mlpH in the phosphate buffered saline solution of 7.2, make blank liposome;
[0084] (2) The blank liposomes prepared above were sterilized by flowing steam, and then ultrasonically treated twice, each time for 20 minutes;
[0085] (3) under aseptic conditions, add ambroxol hydrochloride 30g in the liposome of molten state, add 40g trehalose and 60gPVP under constant stirring;
[0086] (4) Filter through a 0.45 μm microporous membrane, freeze quickly, then return to room temperature, and fill (4ml / bottle) to obtain ambroxol hydrochloride liposome injection.
Embodiment 3
[0087] Example 3 Preparation of Ambroxol Hydrochloride Liposomal Injection
[0088] Prescription: (1000 bottles)
[0089]
[0090] making process
[0091](1) Cholesterol 50g, soybean phosphatidylserine 80g, soybean sterol 30g and 20g Tween 80 are dissolved in the phosphate buffer solution that 2000mlpH is 7.2, make blank liposome;
[0092] (2) The blank liposomes prepared above were sterilized by flowing steam, and then ultrasonically treated twice, each time for 20 minutes;
[0093] (3) under aseptic conditions, add ambroxol hydrochloride 15g in the liposome of molten state, add 20g trehalose and 30gPVP under constantly stirring;
[0094] (4) Filter through a 0.45 μm microporous membrane, fill (2ml / bottle), and directly freeze-dry to obtain ambroxol hydrochloride injection freeze-dried powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com