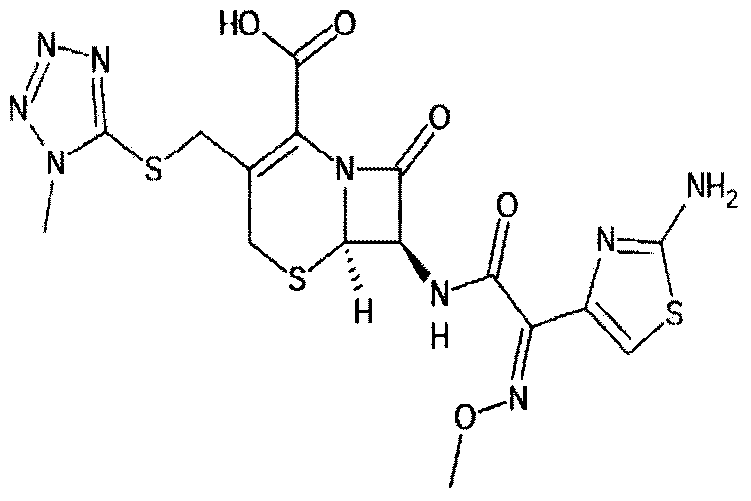
Preparation method of cefmenoxine hydrochloride dry powder
A technology of cefmenoxime hydrochloride and dry powder is applied in the field of preparation of dry drug powder, and can solve the problems of uneven mixing, large static electricity and difficult mixing, poor fluidity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Add 4kg of cefmenoxime hydrochloride to 100L of water, adjust to dissolve with one or more aqueous solutions of sodium carbonate, sodium bicarbonate, and sodium hydroxide, add 0.1kg of activated carbon for decolorization for 15-30 minutes, filter aseptically, and enter In a crystallization tank in a sterile room, add XXX methanol to the filtrate as a dispersant, add dropwise 3M hydrochloric acid to pH 0.5-4.0, precipitate crystals, raise the temperature to 30-45°C, grow crystals for 1-30 minutes, and cool down to 15-29 ℃, grow crystals for 1-30 minutes, then lower the temperature to 0-14°C, grow crystals for 1-3 hours, filter, wash with 5L of water, and vacuum-dry at 35°C for 16 hours to obtain 3.2 kg of sterile cefmenoxime hydrochloride dry powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com