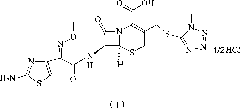
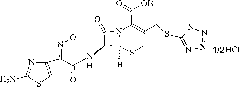
High-purity cefmenoxime hydrochloride compound
A technology of cefmenoxime hydrochloride and a compound, which is applied in the field of medicine, can solve the problems affecting the quality of preparations, poor color, poor purity, etc., and achieves the effects of optimizing product quality, simple process and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The refining of embodiment 1 cefmenoxime hydrochloride
[0018] (1) 100g of cefmenoxime hydrochloride crude product is dissolved in 120ml of water, then slowly add 5% sodium carbonate solution, stir to react to solution pH and be 7, then add the gac of 1.22g, stir at room temperature for 30 minutes, decarburize by filtration, collect filtrate.
[0019] (2) Add 0.1mol / L hydrochloric acid to the filtrate obtained in the upward step to react until the pH value of the solution is 5.5, then add 1000ml of isopropanol, stir and react at room temperature for 30 minutes, filter, and dry under reduced pressure at 45°C to obtain refined cephalosporin hydrochloride 91.4 g of methyloxime, the yield is 91.4%, and the HPLC purity is 99.6%.
[0020] 1 H-NMR MHz(Bruker AV400mHz)(DMSO-d 6 )δ: 9.63(d, 1H, CONH), 6.73(s, 1H, thiazole ring C 5 -H), 5.77 (dd, 1H, C 7 -H), 5.11(d, 1H, C 6 -H), 4.22(q, 2H, C 3 -CH 2 ), 3.93 (s, 3H, -OCH 3 ), 3.84 (s, 3H, -NCH 3 ), 3.72(q, 2H, C 2 -C...
Embodiment 2
[0021] The refining of embodiment 2 cefmenoxime hydrochloride
[0022] (1) 100g cefmenoxime hydrochloride crude product is dissolved in 150ml water, then slowly add 10% sodium bicarbonate solution, stirring reaction is 8 to solution pH, then adds the gac of 2.98g, stirs at room temperature 20 minutes, filter decarburization, Collect the filtrate.
[0023] (2) Add 1 mol / L hydrochloric acid to the filtrate obtained in the upward step to react until the pH value of the solution is 4.5, then add 150 ml of isopropanol, stir and react at room temperature for 60 minutes, filter, and dry under reduced pressure at 50 ° C to obtain refined cephalexin hydrochloride Oxime 92.5g, yield 92.5%, HPLC purity 99.5%.
[0024] 1 H-NMR MHz(Bruker AV400mHz)(DMSO-d 6 )δ: 9.63(d, 1H, CONH), 6.73(s, 1H, thiazole ring C 5 -H), 5.77 (dd, 1H, C 7 -H), 5.11(d, 1H, C 6 -H), 4.22(q, 2H, C 3 -CH 2 ), 3.93 (s, 3H, -OCH 3 ), 3.84 (s, 3H, -NCH 3 ), 3.72(q, 2H, C 2 -CH 2 ).
Embodiment 3
[0025] The refining of embodiment 3 cefmenoxime hydrochloride
[0026] (1) 100g cefmenoxime hydrochloride crude product is dissolved in 150ml water, then slowly add 8% sodium hydroxide solution, stirring reaction is 7.5 to solution pH, then adds the gac of 2.56g, stirs at room temperature 30 minutes, filter decarburization, Collect the filtrate.
[0027] (2) Add 0.5mol / L hydrochloric acid to the filtrate obtained in the upward step to react until the pH value of the solution is 5.0, then add 150ml of isopropanol, stir and react at room temperature for 60 minutes, filter, and dry under reduced pressure at 45°C to obtain refined cephalosporin hydrochloride Methyloxime 91.8g, the yield is 91.8%, and the HPLC purity is 99.6%.
[0028] 1 H-NMR MHz(Bruker AV400mHz)(DMSO-d 6 )δ: 9.63(d, 1H, CONH), 6.73(s, 1H, thiazole ring C 5 -H), 5.77 (dd, 1H, C 7 -H), 5.11(d, 1H, C 6 -H), 4.22(q, 2H, C 3 -CH 2 ), 3.93 (s, 3H, -OCH 3 ), 3.84 (s, 3H, -NCH 3 ), 3.72(q, 2H, C 2 -CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com