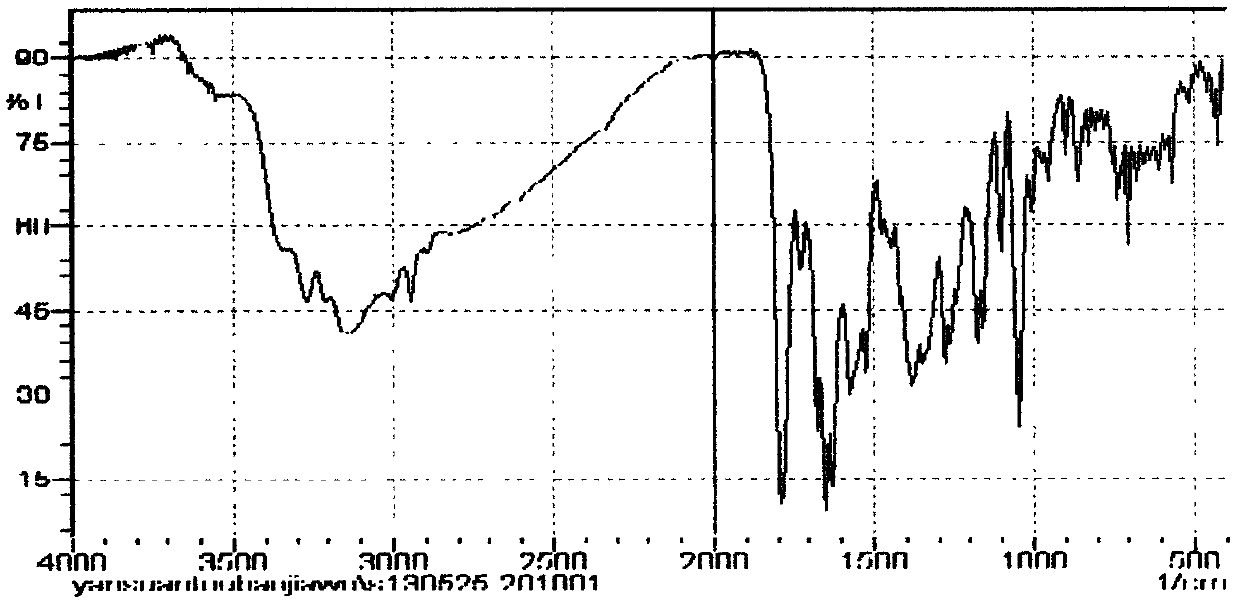
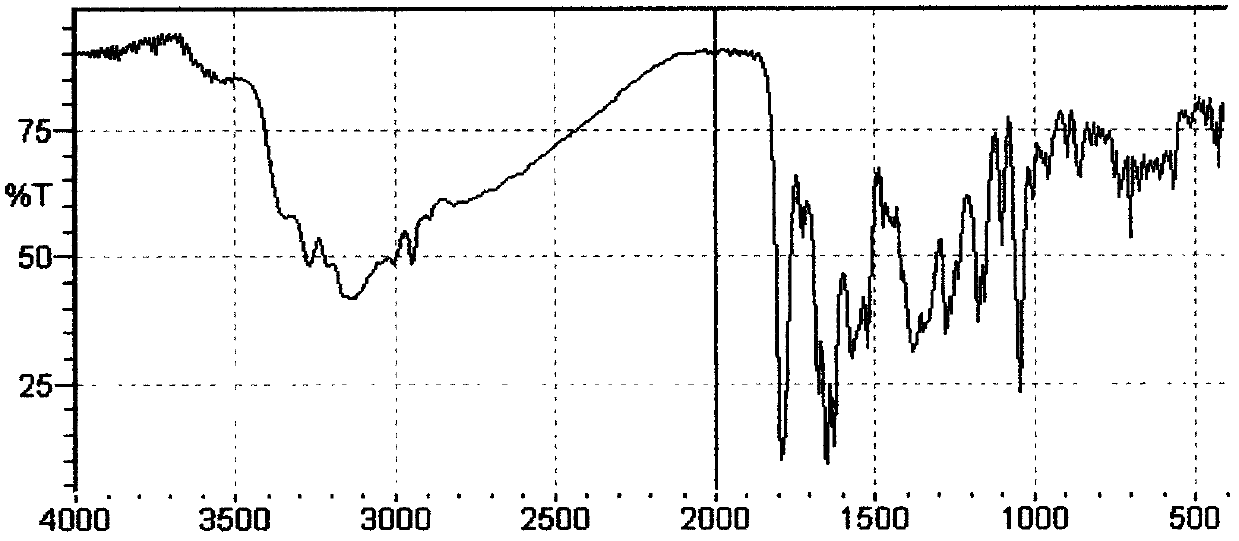
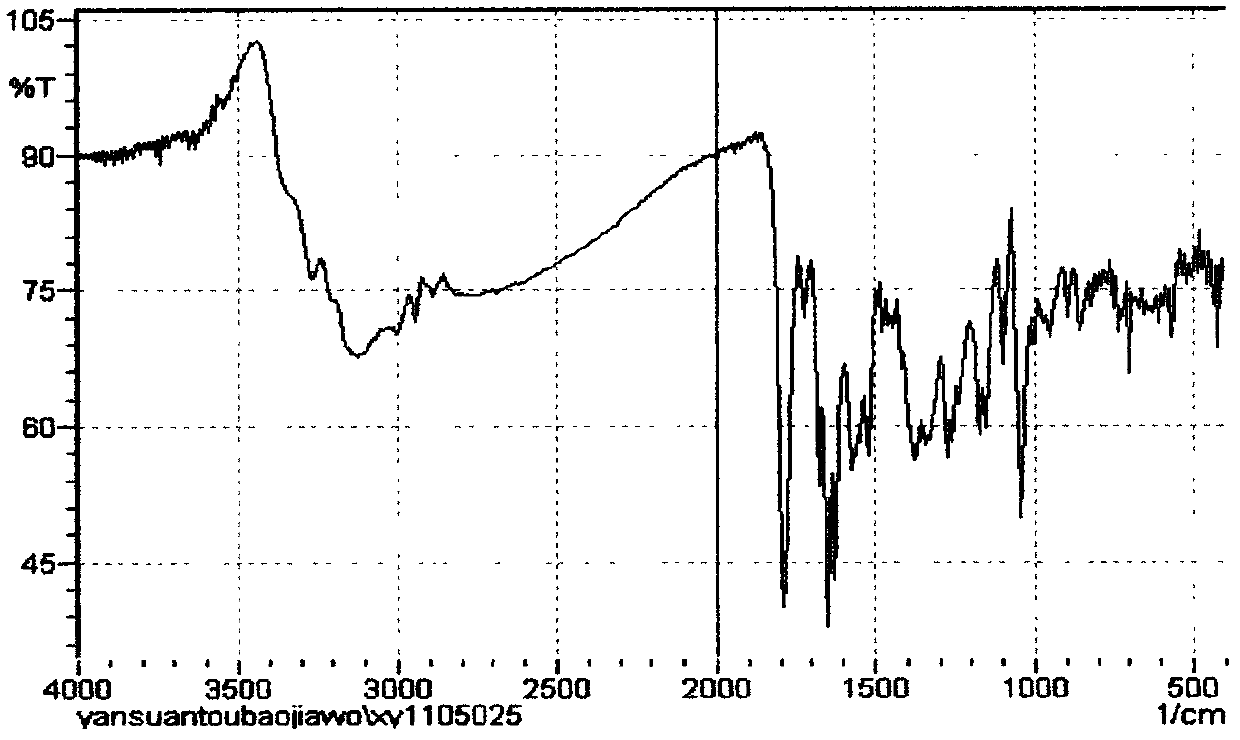
Method for preparing cefmenoxime hydrochloride
A technology of cefmenoxime hydrochloride and hydrochloride, which is applied in the field of compound preparation, can solve the problems of low yield, complicated synthetic route, and high production cost, and achieve the effects of low color grade, high purity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 260ml of dichloromethane, 5ml of ethanol and 35ml of isopropanol into a 500ml four-necked bottle, cool down to -7°C, add 20g of 7-ACT hydrochloride and 21g of active ester of compound AE represented by formula III in sequence, and dropwise add 20ml of triethylamine, After 20 minutes of dripping. At the end of the drop, keep warm at -3°C for 5 hours for condensation reaction. The endpoint was detected by high performance liquid chromatography. After the reaction was complete, 150 ml of anhydrous salt was added, and the layers were separated. The organic phase was extracted once more with 90 ml of anhydrous salt, the aqueous phases were combined, and the aqueous phase was back-extracted with dichloromethane. The pH value of the aqueous phase was adjusted to 6-7 with acid, and 1 g of activated carbon and 0.1 g of sodium bisulfite were added for decolorization for 20 min. Filter and wash the filter cake with 70ml of water. Another 1000ml four-necked bottle was instal...
Embodiment 2
[0029] Add 26L of dichloromethane, 5L of ethanol and 35L of isopropanol to a 50L reaction tank, cool down to -8°C, add 2kg of 7-ACT hydrochloride and 2.1kg of compound AE active ester represented by formula III in sequence, and add 2.0L of triethylamine dropwise , 20min after dripping. At the end of the dropping, keep warm at -4°C for 5 hours for condensation reaction. The endpoint was detected by high performance liquid chromatography. After the reaction was complete, 15 L of anhydrous salt was added, and the layers were separated. The organic phase was extracted once more with 9 L of anhydrous salt, the aqueous phases were combined, and the aqueous phase was back-extracted with dichloromethane. The pH value of the aqueous phase was adjusted to 6-7 with acid, and 0.12 kg of activated carbon and 0.012 kg of sodium bisulfite were added for decolorization for 20 minutes. Filter and wash the filter cake with 7L of water. Another 100L reaction tank was prepared, and 18L of acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com