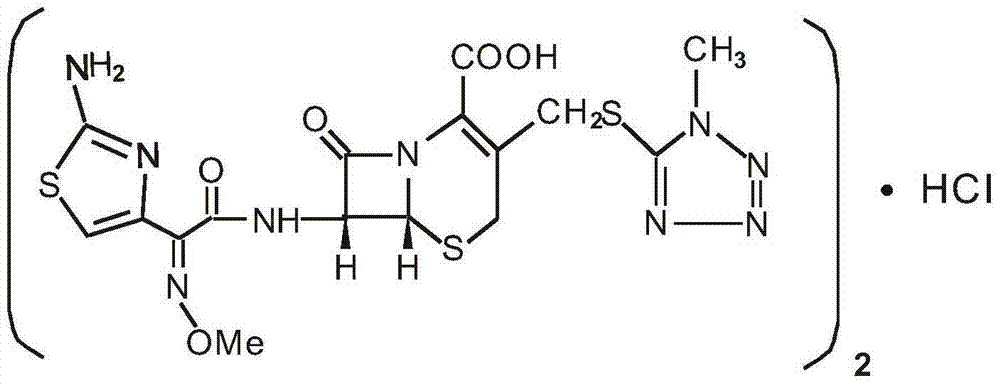
Medicinal composition of cefmenoxime hydrochloride
A technology of cefmenoxime hydrochloride and composition, which is applied in the field of pharmaceutical compositions containing the active ingredient cefmenoxime hydrochloride, and can solve problems such as increased impurities, poor stability, and impact on drug safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Cefmenoxime hydrochloride 1000g, sodium carbonate 533g, ethylenediamine 267g.
[0079] Preparation method: The cefmenoxime hydrochloride raw material is pulverized with a jet pulverizer using low-temperature inert gas (nitrogen) under sterile conditions. The pulverized cefmenoxime hydrochloride and auxiliary materials were mixed in a three-dimensional mixer for 30 minutes until uniformly mixed; the uniformly mixed aseptic mixed powder was packed in sterilized 20ml vials under aseptic conditions, and pressed Stoppering, capping, full inspection, packaging after passing the test, respectively to obtain 1000 bottles of powder injection.
Embodiment 2
[0081] Cefmenoxime hydrochloride 1000g, sodium carbonate 516g, ethylenediamine 284g.
[0082] Preparation method: The cefmenoxime hydrochloride raw material is pulverized with a jet pulverizer using low-temperature inert gas (nitrogen) under sterile conditions. The pulverized cefmenoxime hydrochloride and auxiliary materials were mixed in a three-dimensional mixer for 30 minutes until uniformly mixed; the uniformly mixed aseptic mixed powder was packed in sterilized 20ml vials under aseptic conditions, and pressed Stoppering, capping, full inspection, packaging after passing the test, respectively to obtain 1000 bottles of powder injection.
Embodiment 3
[0084] Cefmenoxime hydrochloride 1000g, sodium carbonate 485g, ethylenediamine 315g.
[0085] Preparation method: The cefmenoxime hydrochloride raw material is pulverized with a jet pulverizer using low-temperature inert gas (nitrogen) under sterile conditions. The pulverized cefmenoxime hydrochloride and auxiliary materials were mixed in a three-dimensional mixer for 30 minutes until uniformly mixed; the uniformly mixed aseptic mixed powder was packed in sterilized 20ml vials under aseptic conditions, and pressed Stoppering, capping, full inspection, packaging after passing the test, respectively to obtain 1000 bottles of powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com