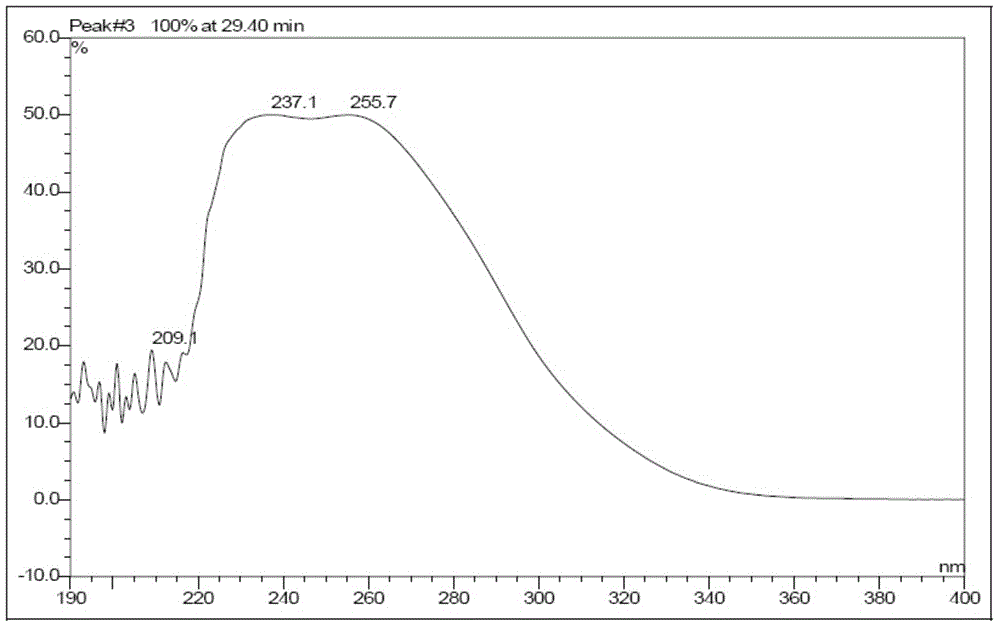
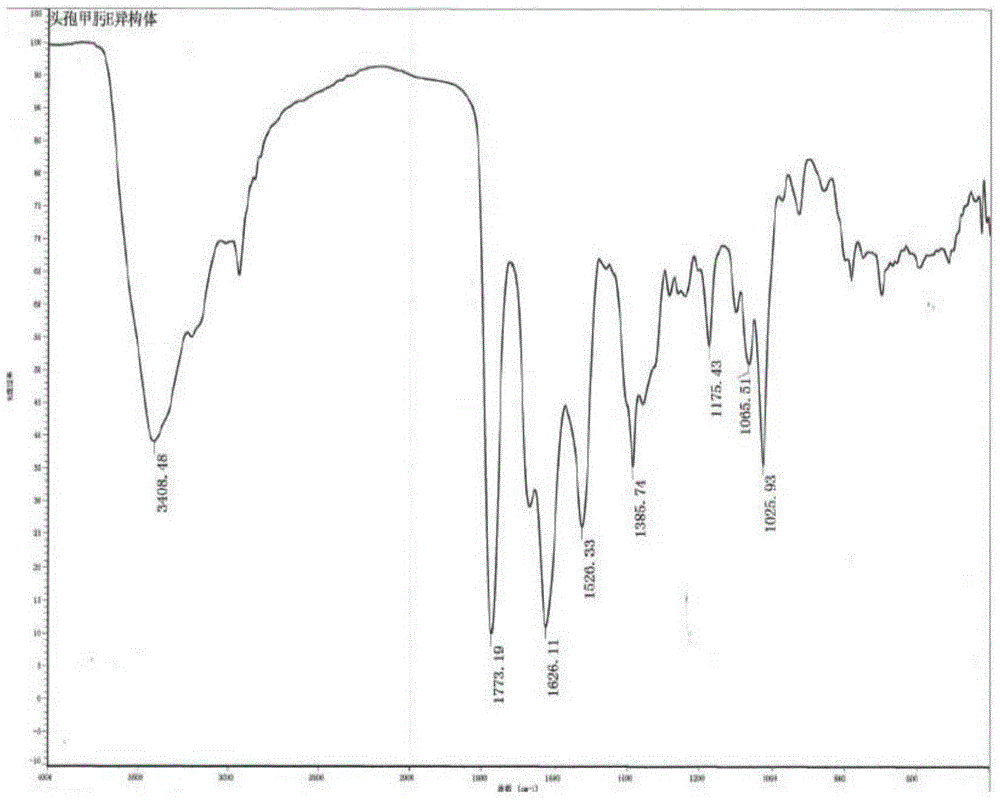
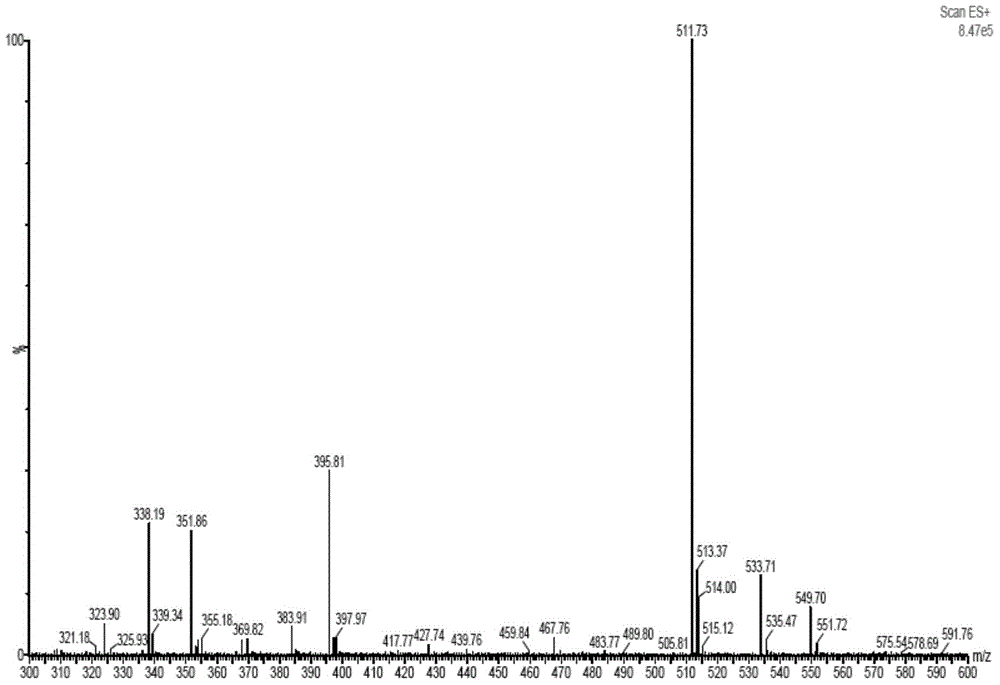
Method of preparing cefmenoxime E isomers
A technology for cefmenoxime and isomers, which is applied in the field of preparation of cefmenoxime E isomers, can solve the problems of not being prepared, unable to confirm the structure and the like, and achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 5.0g of 7-ATCA·HCl and 5.77g of trans-AE active ester into a three-necked flask, add 20ml of DMF, 20ml of THF, and 5ml of purified water. While stirring, 4.8ml of triethylamine was added dropwise until the solution was dissolved. After reacting for 2 hours, add 50ml of water and 30ml of dichloromethane to extract, separate the liquid to take the water phase, add 0.2g of activated carbon, stir for 15min, filter, cool the filtrate to below 15°C, add concentrated hydrochloric acid dropwise to pH2.5~3.0, continue stirring for 1h After filtering, the filter cake was vacuum-dried at 35°C to obtain the E isomer of cefmenoxime.
[0023] The purity was 98% by HPLC.
Embodiment 2
[0025] Weigh 5.0g of 7-ATCA·HCl and 5.30g of trans-AE active ester into a three-necked flask, add 20ml of DMF, 25ml of THF, and 5ml of purified water. While stirring, add 2.8ml of concentrated ammonia water dropwise until the solution is dissolved. After reacting for 1 hour, add 50ml of water and 30ml of dichloromethane for extraction, separate the liquid to take the water phase, add 0.2g of activated carbon, stir for 15 minutes, filter, cool the filtrate to below 15°C, add concentrated hydrochloric acid dropwise to pH 2.5-3.0, and continue stirring for 1 hour After filtering, the filter cake was vacuum-dried at 35°C to obtain the E isomer of cefmenoxime.
[0026] The purity was 98% by HPLC.
Embodiment 3
[0028] Weigh 5.0g of 7-ATCA·HCl and 6.25g of trans-AE active ester into a three-necked flask, add 25ml of DMF, 25ml of THF, and 5ml of purified water. While stirring, 1.5 g of sodium hydroxide was added until the solution was dissolved. After reacting for 3 hours, add 50ml of water and 30ml of dichloromethane to extract, separate the liquid to take the water phase, add 0.1g of activated carbon, stir for 15min, filter, cool the filtrate to below 15°C, add concentrated hydrochloric acid dropwise to pH2.0~3.0, continue stirring for 1h After filtering, the filter cake was vacuum-dried at 35°C to obtain the E isomer of cefmenoxime.
[0029] The purity was 97% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com