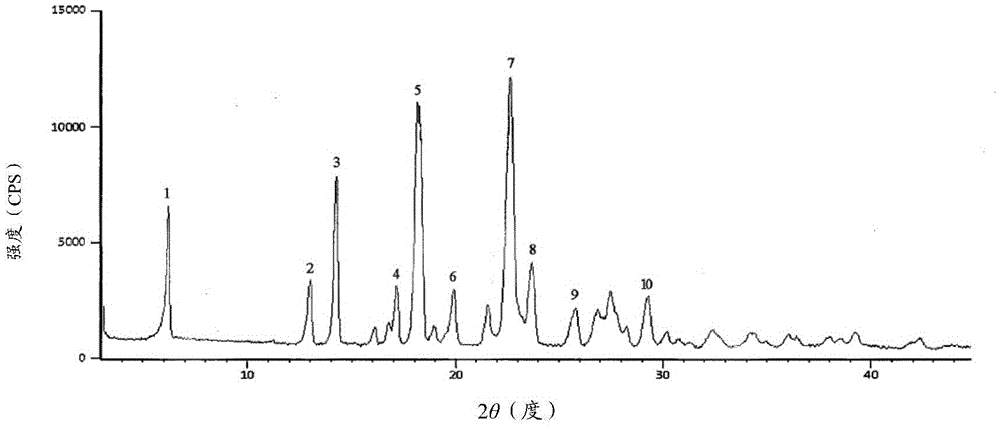
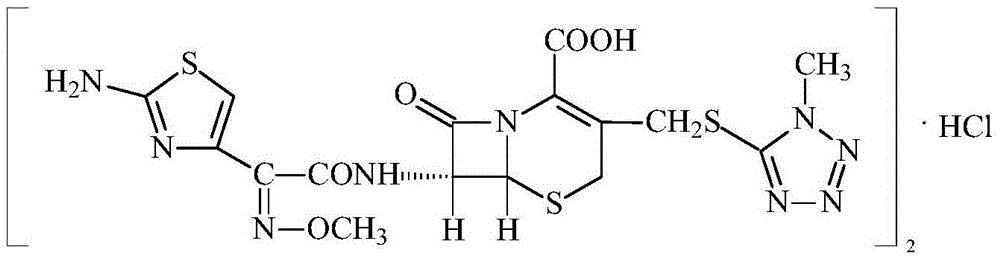
New crystal form cefmenoxine hydrochloride compound prepared by adopting particle process crystal product molecular assembling and morphology optimizing technology and preparation
A technology of cefmenoxime hydrochloride and its compound, applied in the field of medicine, can solve problems such as poor color grade, poor stability of conventional crystal form of cefmenoxime hydrochloride, and occurrence of degradation products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Preparation of Cefmenoxime Hydrochloride New Crystal Form Compound
[0024] (1) Add 250ml of dichloromethane, 25ml of ethanol, (6R,7R)-7-amino-3-[[(1-methyl-1-H-tetrazol-5-yl)sulfur into the reaction bottle Substitute]methyl]-8-oxo-5-thia·1-azabicyclo[4,2,0]oct-2-ene-2-carboxylate hydrochloride (7-ATCA·HCl) 25g, 20ml of triethylamine, after reacting at room temperature for 30min, add 50.0g of 2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetic acid thiobenzothiazolyl ester (AE active ester), and control the appropriate React at temperature for 8 hours, add water to extract twice, 150ml each time, combine the water phases, decolorize with activated carbon at room temperature, filter, wash the carbon layer with ethanol, combine the washings, adjust the pH value to 1.5 with 6mol / L hydrochloric acid, grow crystals for 3 hours, filter , washed the filtrate with water, and dried under vacuum at 40°C to obtain crude cefmenoxime hydrochloride.
[0025] (2) Add 25.9 g...
Embodiment 2
[0032] Embodiment 2: Preparation of new crystal form compound of cefmenoxime hydrochloride
[0033] (1) Add 250ml of dichloromethane, 25ml of ethanol, (6R,7R)-7-amino-3-[[(1-methyl-1-H-tetrazol-5-yl)sulfur into the reaction bottle Substitute]methyl]-8-oxo-5-thia·1-azabicyclo[4,2,0]oct-2-ene-2-carboxylate hydrochloride (7-ATCA·HCl) 25g, 20ml of triethylamine, after reacting at room temperature for 30min, add 25.0g of 2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetic acid thiobenzothiazolyl ester (AE active ester), and control the appropriate React at temperature for 8 hours, add water to extract twice, 150ml each time, combine the water phases, decolorize with activated carbon at room temperature, filter, wash the carbon layer with ethanol, combine the washings, adjust the pH value to 2.0 with 8mol / L hydrochloric acid, grow crystals for 3 hours, filter , washed the filtrate with water, and dried under vacuum at 40°C to obtain crude cefmenoxime hydrochloride.
[0034] (2) Add 25....
Embodiment 3
[0036]Embodiment 3: Preparation of new crystal form compound of cefmenoxime hydrochloride
[0037] (1) Add 250ml of dichloromethane, 25ml of ethanol, (6R,7R)-7-amino-3-[[(1-methyl-1-H-tetrazol-5-yl)sulfur into the reaction bottle Substitute]methyl]-8-oxo-5-thia·1-azabicyclo[4,2,0]oct-2-ene-2-carboxylate hydrochloride (7-ATCA·HCl) 25g, 20ml of triethylamine, after reacting at room temperature for 30min, add 75.0g of 2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetic acid thiobenzothiazolyl ester (AE active ester) to control the appropriate React at temperature for 8 hours, add water to extract twice, 150ml each time, combine the water phases, decolorize with activated carbon at room temperature, filter, wash the carbon layer with ethanol, combine the washings, adjust the pH value to 2.0 with 5mol / L hydrochloric acid, grow crystals for 3 hours, filter , washed the filtrate with water, and dried under vacuum at 40°C to obtain crude cefmenoxime hydrochloride.
[0038] (2) Add 23.7 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com