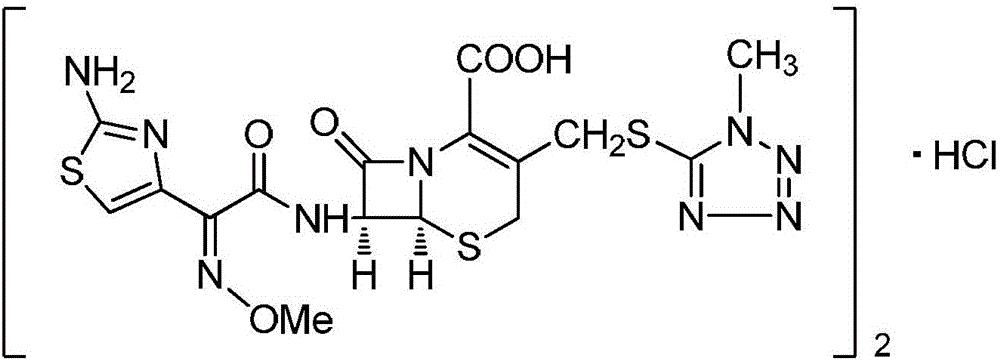
Method for preparing cefmenoxine hydrochloride raw material
A technology of cefmenoxime hydrochloride and raw materials, applied in the field of preparation of cefmenoxime hydrochloride, can solve problems such as poor solubility, endangering life safety, shock, death and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
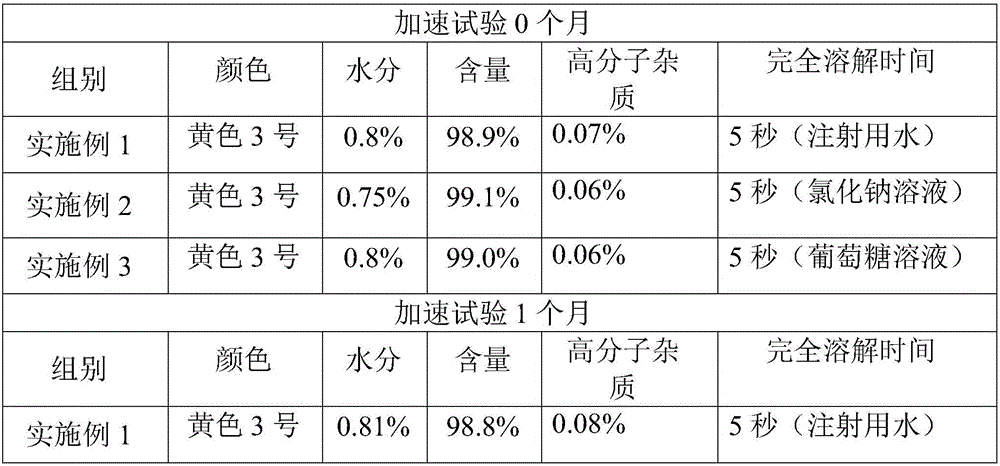
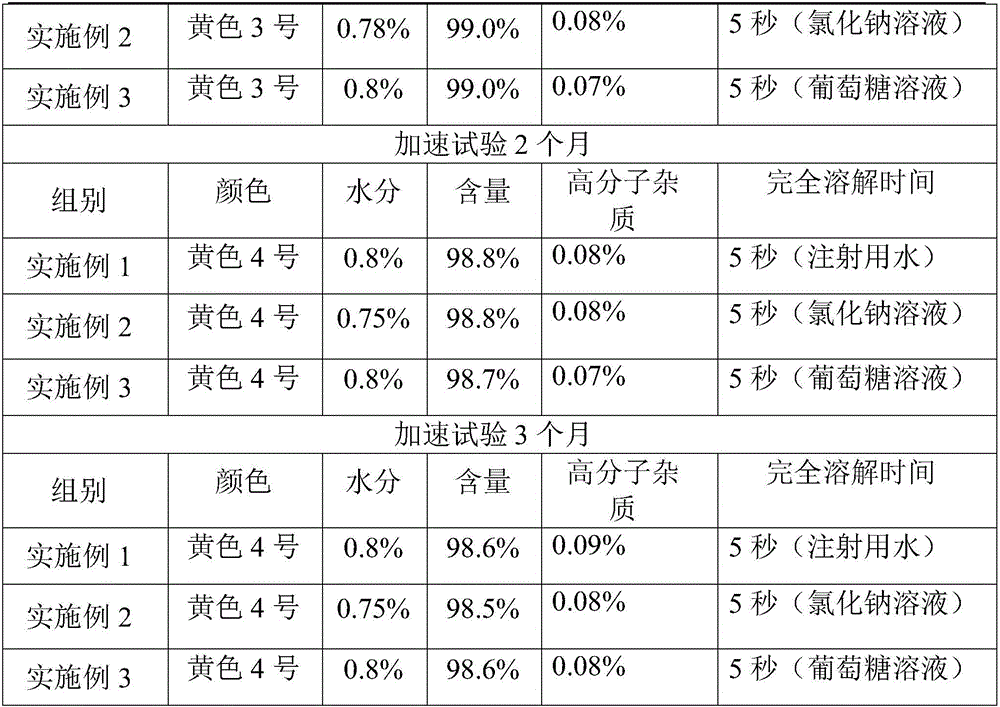
[0021] Take 100g of 7-amino-3-[1-methyl-1H-tetrazole-5-thiomethyl]-ceph-3-ene-4-carboxylic acid (7-ACT), 1000ml of dichloromethane, and cool in an ice-water bath To about 5°C, add 120g of AE active ester, then add 100ml of triethylamine, continue to stir for 4 hours, extract twice with 500ml of water, combine the water phase, add 10g of activated carbon, stir for 30 minutes to decolorize, filter with suction, and wash with 200ml of water Carbon cake, combined water phase. The filtrate was adjusted to pH 2.5 with 10% hydrochloric acid, and a white solid was precipitated, stirred and grown for 3 hours, and filtered. Wash twice with 500ml ethanol and drain. Dry under reduced pressure at a temperature lower than 40°C for 10 hours until the water content is lower than 1.0%, to obtain 82.5 g of crude cefmenoxime hydrochloride.
[0022] Step 2: Take 50g of crude cefmenoxime hydrochloride, add 200mL of water for injection, add 5g of L-glutamic acid, heat up to 38°C, add 1g of dietha...
Embodiment 2
[0024] Take 100g of 7-amino-3-[1-methyl-1H-tetrazole-5-thiomethyl]-ceph-3-ene-4-carboxylic acid (7-ACT), 1000ml of dichloromethane, and cool in an ice-water bath At about 5°C, add 120g of AE active ester, then add 100ml of triethylamine, continue to stir for 4 hours, extract twice with 500ml of water, combine the water phase, add 10g of activated carbon, stir for 30 minutes to decolorize, filter with suction, and wash with 200ml of water Carbon cake, combined water phase. The filtrate was adjusted to pH 2.5 with 10% hydrochloric acid, and a white solid was precipitated, stirred and grown for 3 hours, and filtered. Wash twice with 500ml ethanol and drain. Dry under reduced pressure at a temperature lower than 40°C until the water content is lower than 1.0%, to obtain 83.2 g of crude cefmenoxime hydrochloride.
[0025] Step 2: Take 50g of cefmenoxime hydrochloride crude product, add 200mL of water for injection, add 5g of L-glutamic acid, heat up to 35°C, add 1g of diethanolam...
Embodiment 3
[0027] Take 100g of 7-amino-3-[1-methyl-1H-tetrazole-5-thiomethyl]-ceph-3-ene-4-carboxylic acid (7-ACT), 1000ml of dichloromethane, and cool in an ice-water bath At about 5°C, add 120g of AE active ester, then add 100ml of triethylamine, continue to stir for 4 hours, extract twice with 500ml of water, combine the water phase, add 10g of activated carbon, stir for 30 minutes to decolorize, filter with suction, and wash the carbon with 200ml of water cake, and combine the aqueous phases. The filtrate was adjusted to pH 2.5 with 10% hydrochloric acid, and a white solid was precipitated, stirred and grown for 3 hours, and filtered. Wash twice with 500ml ethanol and drain. Dry under reduced pressure at a temperature lower than 40°C until the water content is lower than 1.0%, to obtain 82.1 g of crude cefmenoxime hydrochloride.
[0028] Step 2: Take 50g of crude cefmenoxime hydrochloride, add 200mL of water for injection, add 5g of L-glutamic acid, heat up to 38°C, add 1g of dieth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com