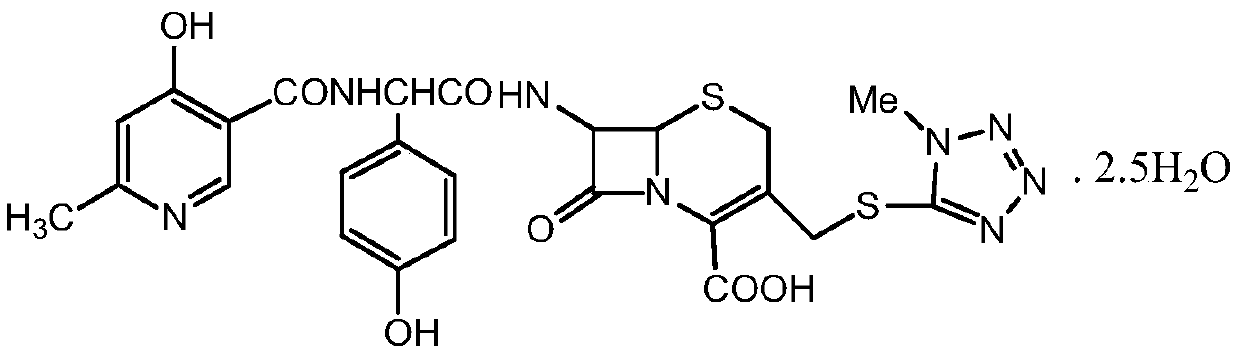
Purification method of cefpiramide acid
A technology of cefpiramide and a refining method, which is applied in the refining field of cefpiramide, can solve the problems of poor yield and low purity of cefpiramide, and achieves low production cost, high yield, and simple operation in the preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The present embodiment provides a kind of refining method of cefpiramide, comprising the following steps:
[0030] Step a, add 340L methanol and 80Kg cefpiram acid crude product (TPC) to R-125 reaction kettle, drop triethylamine 20.96Kg under the condition of 10 ℃, dropwise time is 30min, obtains cefpiram acid three Ethylamine salt solution;
[0031] Step b, add 1200L of acetone to the cefpiramide salt solution at 20°C, stir at a speed of 300rpm for 1h, then cool down to 10°C for centrifugal filtration at a speed of 800rpm, and wash with 150L of acetone, Dried time is 30min, obtains cefpiramic acid triethylamine salt (TPT);
[0032] Step c, add 600L of purified water to the R-225 reactor, and add the cefpiramic acid triethylamine salt obtained in step b, mix at 10°C for 10min, then raise the temperature to 15°C and add 5Kg of activated carbon for decolorization for 20min, Filter to remove carbon, collect the filtrate into the crystallization tank R-325, and add 2M hyd...
Embodiment 2
[0034] The present embodiment provides a kind of refining method of cefpiramide, comprising the following steps:
[0035] Step a, add 160L ethanol and 80Kg cefpiramic acid crude product (TPC) in R-125 reactor, drop triethylamine 22.1Kg under the condition of 12 ℃, dropwise time is 25min, obtains cefpiramic acid three Ethylamine salt solution;
[0036] Step b. Add 960L of isopropanol to the cefpiramide salt solution at 18°C, stir for 70min at a speed of 200rpm, then cool down to 15°C for centrifugal filtration at a speed of 700rpm, using 160L isopropanol Propanol washing, drying time is 60min, to obtain cefpiramide triethylamine salt (TPT);
[0037] Step c, add 640L of purified water to the R-225 reaction kettle, and add the cefpiramic acid triethylamine salt obtained in step b, mix at 12°C for 8 minutes, then raise the temperature to 18°C and add 4Kg of activated carbon for decolorization for 25 minutes, Filter to remove carbon, collect the filtrate into the crystallizatio...
Embodiment 3
[0039] The present embodiment provides a kind of refining method of cefpiramide, comprising the following steps:
[0040] Step a, add 200L methanol and 80Kg cefpiram acid crude product (TPC) to the R-125 reaction kettle, add 17.8Kg of triethylamine dropwise under the condition of 13°C, and the dropping time is 32min to obtain cefpiramic acid tri Ethylamine salt solution;
[0041] Step b, add 1360L propylene glycol to the cefpiramide salt solution at 22°C, stir at a speed of 250rpm for 50min, then cool down to 12°C for centrifugal filtration at 750rpm, wash with 120L propylene glycol, The spin-drying time is 40min to obtain cefpiramic acid triethylamine salt (TPT);
[0042] Step c. Add 560L of purified water to the R-225 reactor, and add the cefpiramic acid triethylamine salt obtained in step b, mix at 15°C for 10 minutes, then raise the temperature to 20°C and add 5.6Kg of activated carbon for decolorization for 30 minutes , filter to remove carbon, collect the filtrate into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com