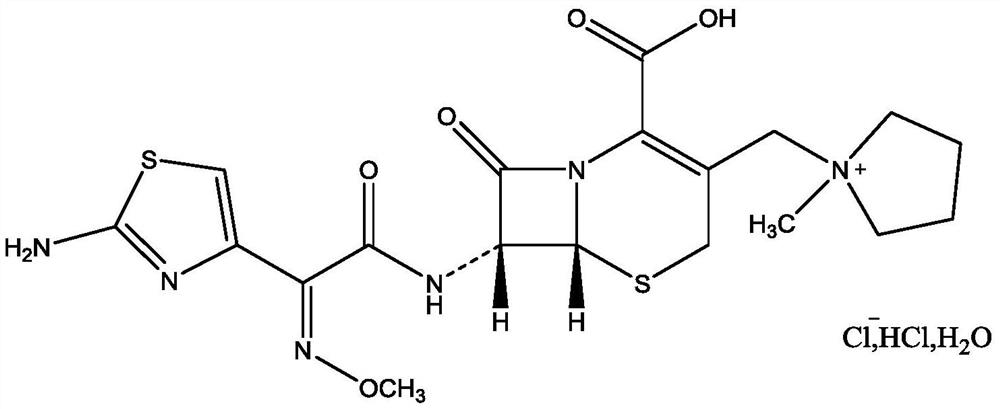
A kind of purification method of cefepime hydrochloride
A technology of cefepime hydrochloride and purification method, applied in the direction of organic chemistry, etc., to achieve the effect of saving production cost, ensuring stability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method comprises the following steps:
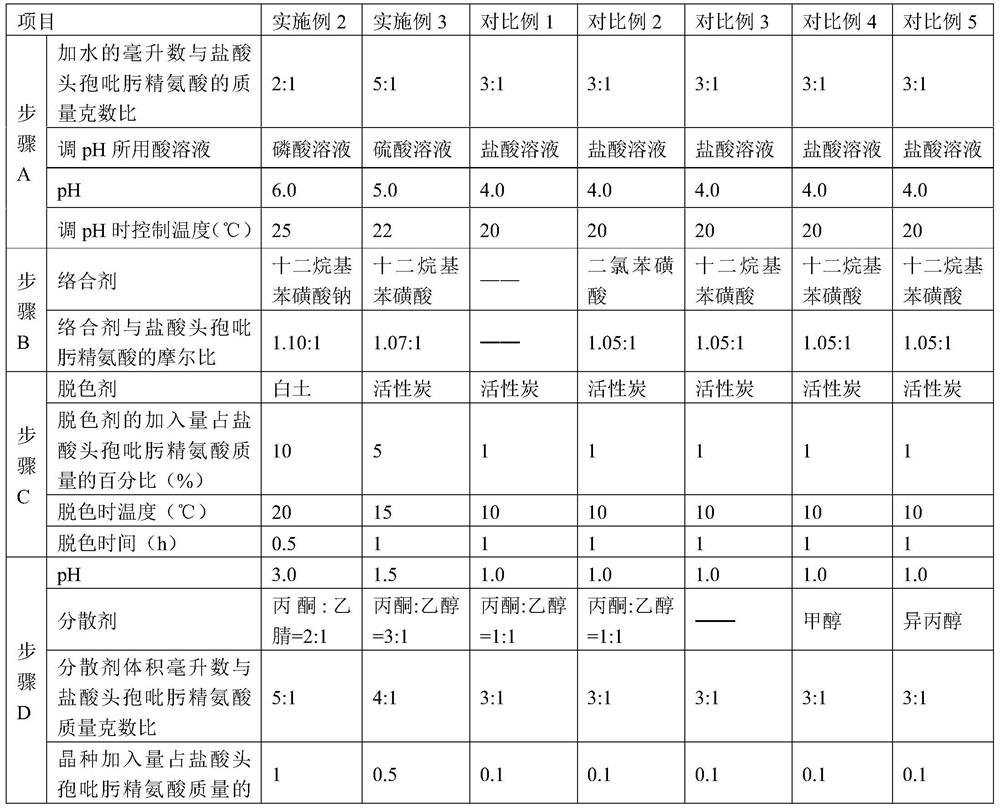
[0033] A. Add water to dissolve cefepime hydrochloride arginine, control the temperature at 20-25°C, adjust the pH to 4.0-6.0 with hydrochloric acid, phosphoric acid or sulfuric acid solution, stir and dissolve to obtain cefepime hydrochloride arginine solution ;
[0034] The ratio of the milliliters of water added to the mass grams of cefepime hydrochloride arginine is 2 to 5:1;
[0035] B. add complexing agent in cefepime hydrochloride arginine dissolving solution, filter and remove precipitate, obtain filtrate, wash complex compound with water, obtain washing solution;
[0036] The complexing agent is sodium dodecylbenzenesulfonate or dodecylbenzenesulfonic acid, and the molar ratio of complexing agent to cefepime hydrochloride arginine in cefepime hydrochloride is 1.05~1.10:1;
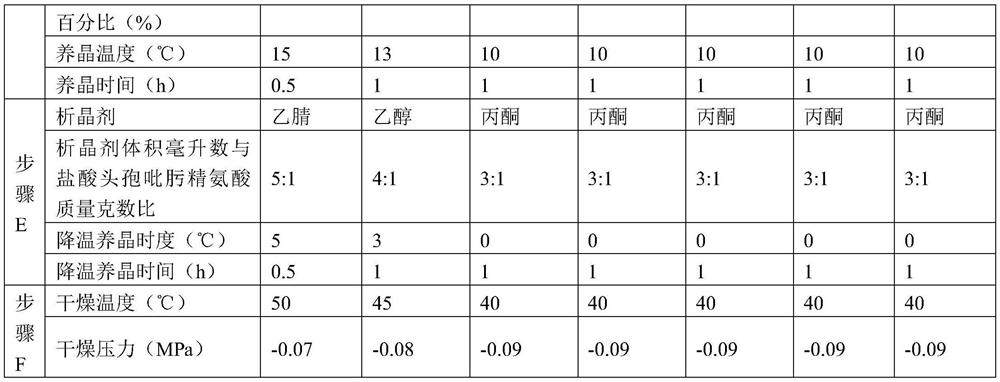
[0037] C. Combine the filtrate and washing liquid, add decolorizing agent, the decolorizing temperature is 10-20°C, decolorize for 0...
Embodiment 1
[0045] A method for purifying cefepime hydrochloride, using cefepime hydrochloride arginine hydrochloride that does not meet the quality index of cefepime hydrochloride for injection in the 2015 edition of the Chinese Pharmacopoeia as a raw material, adding cefepime hydrochloride arginine hydrochloride solution The complexing agent is used for decolorization, and then a dispersant and a crystallization agent are added to the decolorization solution for crystallization to obtain cefepime hydrochloride.
[0046] The preparation method comprises the following steps:
[0047] A. Add 60mL of water to dissolve in 20g cefepime hydrochloride arginine (containing cefepime 12.6g), adjust the pH to 4.0 with 18% hydrochloric acid solution, control the stirring temperature to be 20°C, stir and dissolve until dissolved to get cephalosporin hydrochloride Pyroxime arginine solution;
[0048] B. Add 9.0 g of dodecylbenzenesulfonic acid to the raw material solution, react for 2 hours, remove t...
Embodiment 2~3
[0054] Embodiments 2-3 have the same production process steps as Embodiment 1, the difference is the selection of process parameters, as shown in Table 1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com