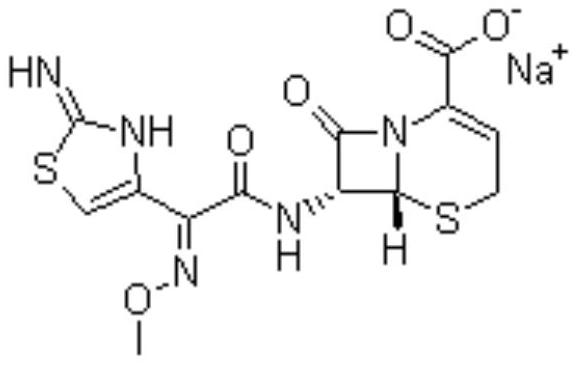
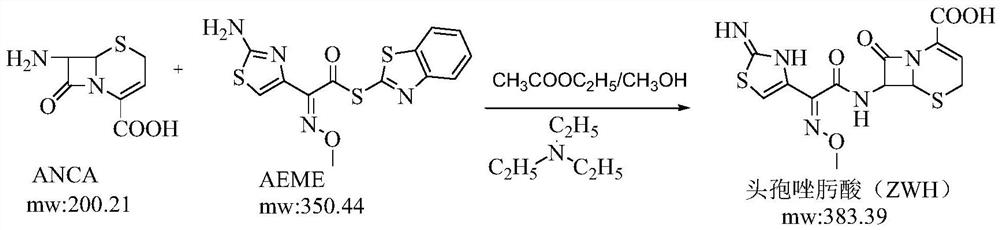
Method for synthesizing ceftizoxime acid by one-pot method
A technology of ceftizoxamic acid and tetrahydrofuran, which is applied in the field of pharmaceutical and chemical technology synthesis, can solve the problems of complex synthesis steps of ceftizoxamic acid and difficult solvent recovery and application, and achieve the effect of mild process conditions, easy operation and low color grade
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Take 40ml of purified water and 130ml of tetrahydrofuran to prepare a solvent, and cool the solvent to below 5°C; add 20g of ANCA (0.10mol), 42g of AE-active ester (0.12mol), and rinse the bottle wall with 10ml of tetrahydrofuran, and control the temperature at 0°C. Add 12.1 g (0.12 mol) of triethylamine and 20 ml of tetrahydrofuran mixture dropwise therein, and finish dropping in 60 minutes at a temperature controlled below 5°C, and react for 4 hours at a temperature controlled at -5°C to 5°C to obtain a reaction liquid; take a small amount of the reaction liquid and use HPLC The residual amount of ANCA was measured by the method, and the residual amount of ANCA in the reaction solution was measured to be 0.29 mg / ml.
[0032] (2) Add 3 mol / L hydrochloric acid solution dropwise to the reaction solution, adjust the pH value of the reaction solution to 2.48, and grow crystals at a temperature of 10-15° C. for 30 minutes.
[0033] (3) Filter the solution in the above s...
Embodiment 2
[0037] (1) Take 40ml of purified water and 170ml of tetrahydrofuran to make a solvent, cool the solvent to below 5°C, add 20g of ANCA (0.10mol), 42g of AE-active ester (0.12mol), rinse with 10ml of tetrahydrofuran, control the temperature at 0°C, and drop into it Add 12.1g (0.12mol) of triethylamine and 20ml of tetrahydrofuran mixed solution, drop it in 60min under temperature control below 5°C, control temperature at -5~10°C, react for 5h to obtain reaction solution; take a small amount of reaction solution and measure ANCA by HPLC method The residual amount of ANCA measured in the reaction solution was 0.41 mg / ml.
[0038] (2) Add 3 mol / L hydrochloric acid solution dropwise to the reaction solution, adjust the pH value of the reaction solution to 2.63, and grow crystals at a temperature of 10-15° C. for 30 minutes.
[0039] (3) Filter the solution in the above step (2) to obtain a filter cake, wash the filter cake with 50ml of tetrahydrofuran, 50ml of purified water, and 100...
Embodiment 3
[0042] (1) Take 40ml of purified water and 90ml of tetrahydrofuran to make a solvent, cool the solvent to below 5°C, add 20g of ANCA (0.10mol), 42g of AE-active ester (0.12mol), rinse with 10ml of tetrahydrofuran, control the temperature at 0°C, and drop into it Add 12.1g (0.12mol) of triethylamine (0.12mol) and 20ml of tetrahydrofuran mixed solution, control the temperature below 5°C in 60min, drop it over 60min, control the temperature at -10~5°C, and react for 5h to obtain the reaction solution; take a small amount of the reaction solution and measure ANCA by HPLC The residual amount of ANCA measured in the reaction solution was 0.48mg / ml.
[0043] (2) Add 3 mol / L hydrochloric acid solution dropwise to the reaction solution, adjust the pH value of the reaction solution to 2.33, and grow crystals at a temperature of 10-15° C. for 30 minutes.
[0044] (3) Filter the solution in the above step (2) to obtain a filter cake, wash the filter cake with 50ml tetrahydrofuran, 50ml pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com