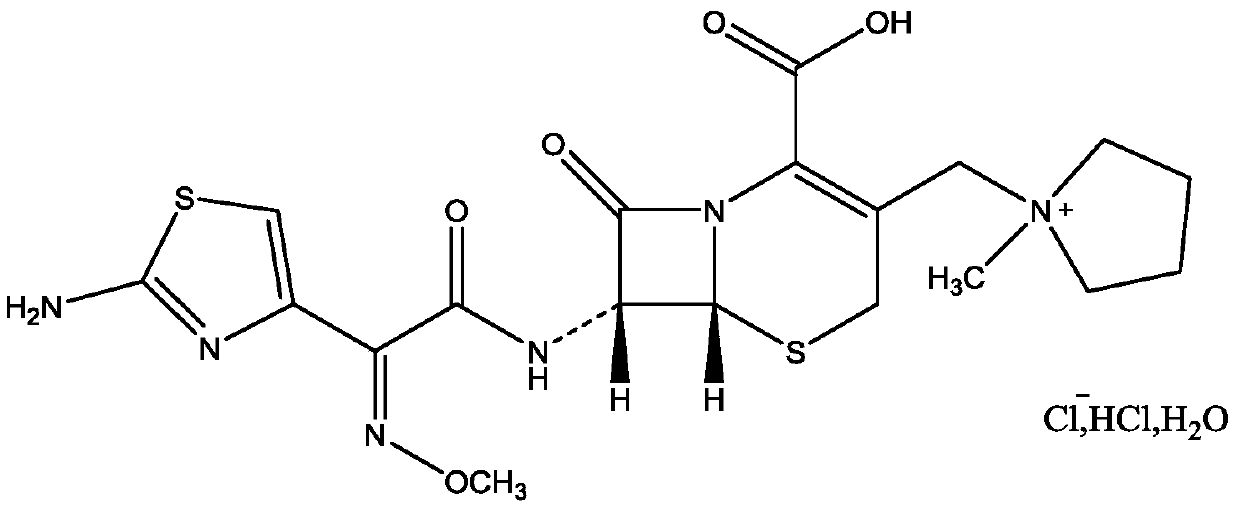
Purification method of cefepime dihydrochloride
A technology of cefepime hydrochloride and purification method, applied in the direction of organic chemistry, etc., to achieve the effects of good stability, high content, and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method includes the following steps:
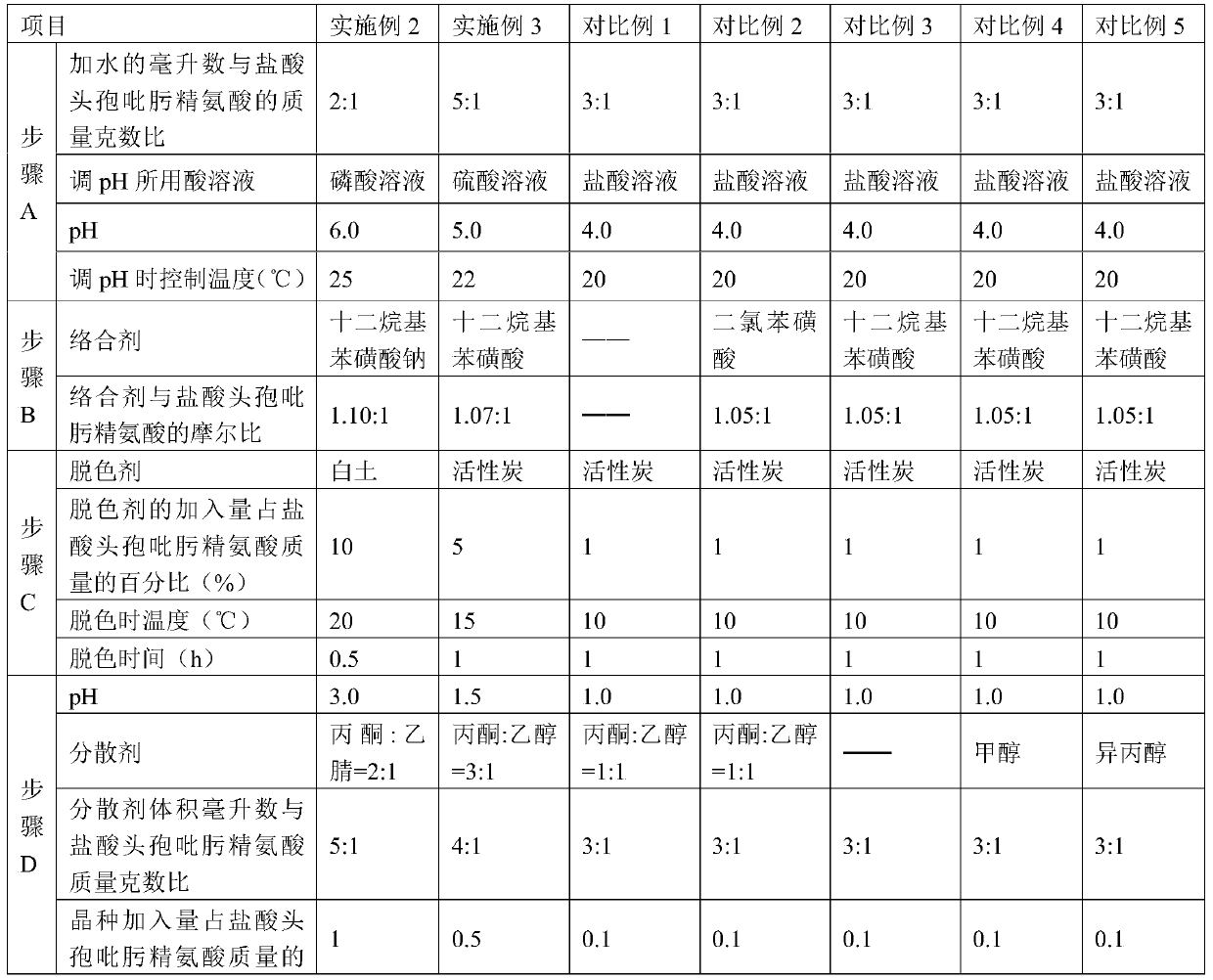
[0033] A. Dissolve cefepime hydrochloride arginine with water, control the temperature at 20-25°C, adjust the pH to 4.0-6.0 with any of hydrochloric acid, phosphoric acid or sulfuric acid solution, stir and dissolve to obtain cefepime hydrochloride arginine solution ;
[0034] The ratio of the number of milliliters of water added to the mass grams of cefepime hydrochloride arginine is 2~5:1;
[0035] B. Add a complexing agent to the cefepime hydrochloride arginine dissolving solution, filter to remove the precipitate to obtain a filtrate, and wash the complex with water to obtain a washing solution;
[0036] The complexing agent is sodium dodecyl benzene sulfonate or dodecyl benzene sulfonic acid, and the molar ratio of the complexing agent to cefepime in cefepime hydrochloride arginine is 1.05~1.10:1;
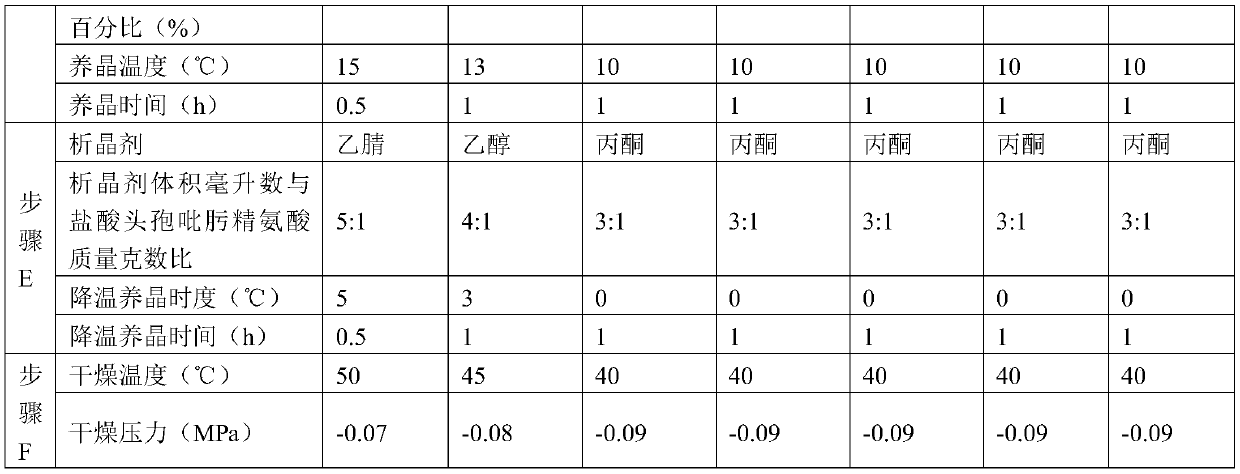
[0037] C. Combine the filtrate and the washing liquid, add a decolorizing agent, the decolorizing temperature is 10-20 ℃, ...
Embodiment 1
[0045] A method for purifying cefepime hydrochloride, using cefepime hydrochloride arginine hydrochloride that does not meet the quality index of cefepime hydrochloride for injection in the 2015 Chinese Pharmacopoeia as a raw material, and adding cefepime hydrochloride arginine hydrochloride dissolving solution After the complexing agent is decolorized, a dispersing agent and a crystallization agent are added to the decolorizing solution for crystallization to obtain cefepime hydrochloride.
[0046] The preparation method includes the following steps:
[0047] A. Add 60mL of water to 20g of cefepime hydrochloride arginine (containing 12.6g of cefepime), adjust the pH to 4.0 with 18% hydrochloric acid solution, control the stirring temperature to 20°C, stir and dissolve until it is clear to obtain cefepime hydrochloride Pyromoxime arginine dissolving solution;
[0048] B. Add 9.0 g of dodecylbenzene sulfonic acid to the raw material solution, react for 2 hours, filter to remove the c...
Embodiment 2~3
[0054] The production process steps in Examples 2 to 3 are the same as those in Example 1, except for the selection of process parameters, as shown in Table 1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com