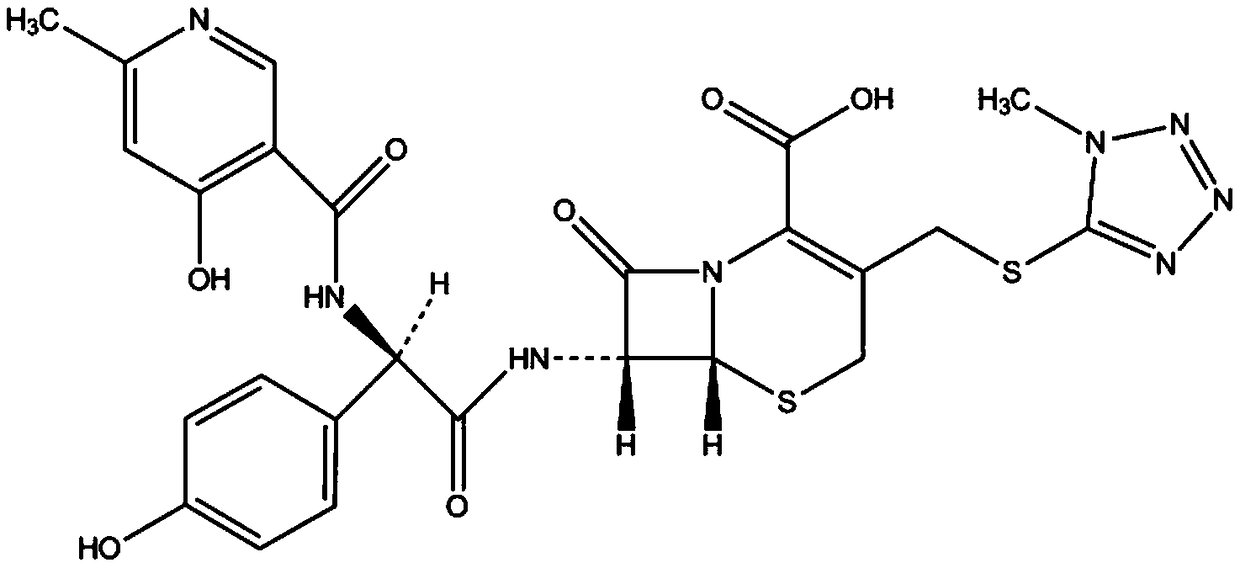
Method for refining cefpiramide acid
A technology of cefpiramide and a purification method, applied in the field of medicine, can solve the problems of unfavorable industrialized production, low weight yield and high production cost, and achieve the effects of effective recycling, high purity and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A method for refining cefpiramin, the specific method for refining is as follows:
[0034] First put 20g of cefpiramic acid crude raw material into the mixed solution of 30ml of methanol and 30ml of dichloromethane, then add 3.2ml of triethylamine, control the temperature at 15°C, stir to dissolve, then add 0.2g of activated carbon for decolorization for 0.5h, filter Collect the filtrate; control the temperature of the filtrate at 10°C, add 100ml of dichloromethane, separate out cefpiramide salt solid crystals, filter; put the solid crystals into a mixed solution of 100ml water and 100ml isopropanol, control the temperature at 10°C, and add 20% Adjust the pH value to 2.0 with hydrochloric acid, add 20mg of seed crystals, precipitate cefpiramic acid, grow the crystals for 0.5h, filter to obtain cefpiramic acid solid crystals and mother liquor; wash cefpiramic acid solid crystals with 100ml of isopropanol, and dry at 40°C , the pressure is -0.09~-0.07MPa during drying, an...
Embodiment 2
[0037] The production process steps in this embodiment are the same as in Example 1. The difference is that the mother liquor is applied mechanically in this embodiment, specifically for dissolving the cefpiramide salt solid crystallization with the mother liquor preserved in Example 1, and all the other process parameters are the same as those in Example 1. Example 1 is the same.
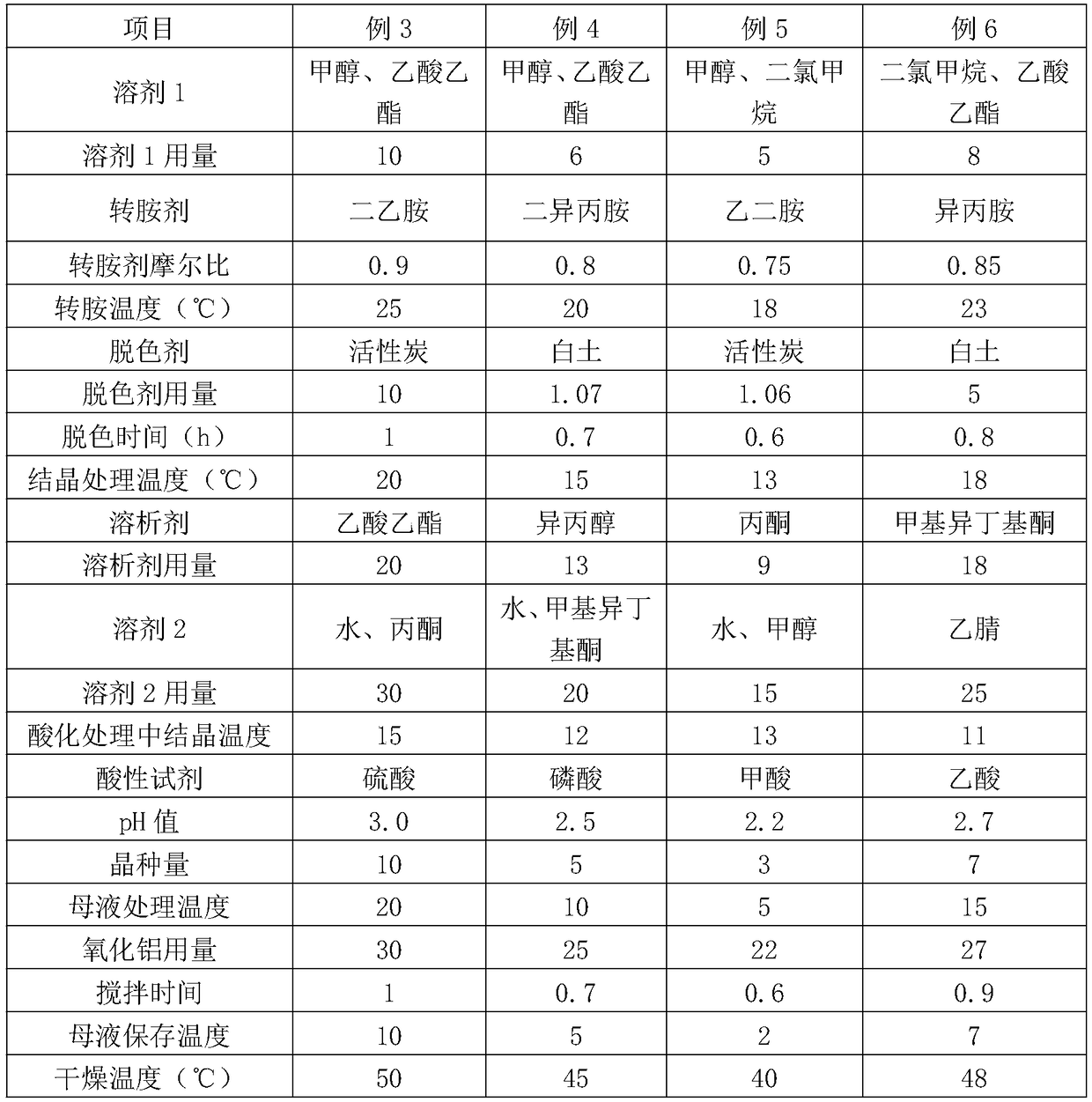
Embodiment 3~6
[0039] Embodiments 3-6 are identical to the production process steps in Embodiment 1, and the difference is the selection of process parameters, as shown in Table 1, wherein:
[0040] "Amount of solvent 1" is the number of milliliters of solvent 1 added to the number of times the weight of the raw material in grams;
[0041] "Molar ratio of transamination agent" refers to the molar ratio of transamination agent to cefpiramin crude product;
[0042] "Depigmentation agent dosage" is the percentage by weight that depigmentation agent weight accounts for cefpiramide crude product;
[0043] "Dissolving agent dosage" is how many times the number of milliliters of dissolving agent is the weight of cefpiramin crude product in grams;
[0044] "Amount of solvent 2" is how many milliliters of solvent 2 is added to the number of grams of solid crystal weight obtained in step C;
[0045] "Amount of seed crystals" is the percentage by weight of the seed crystal weight in the cefpiramin cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com