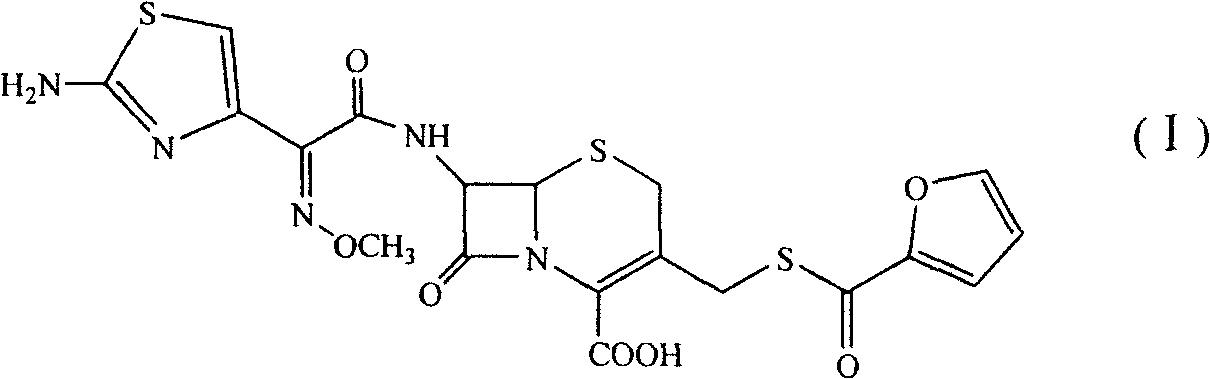
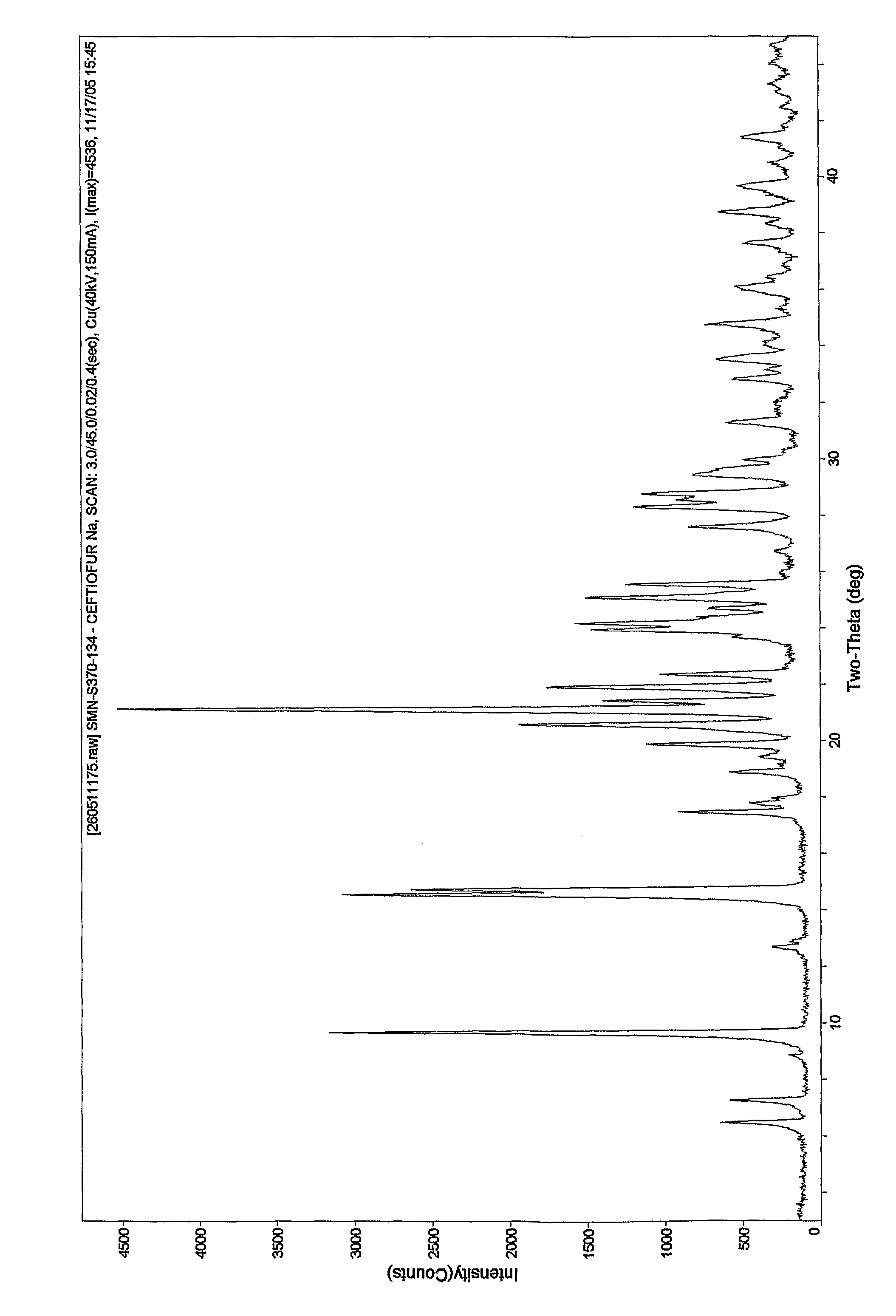
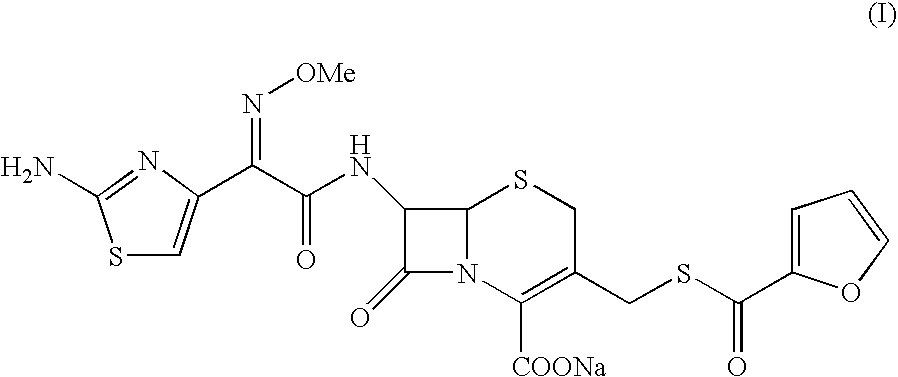
Patents
Literature
84 results about "Ceftiofur sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
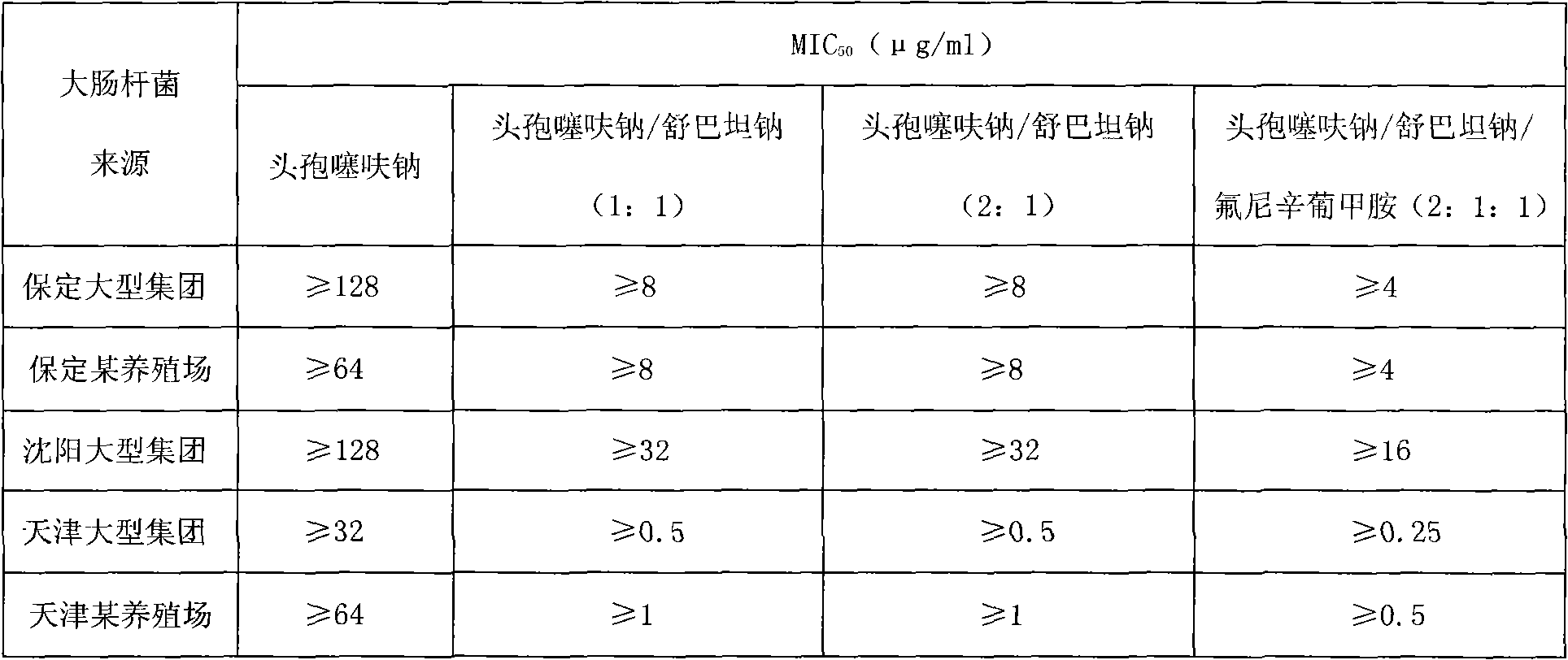
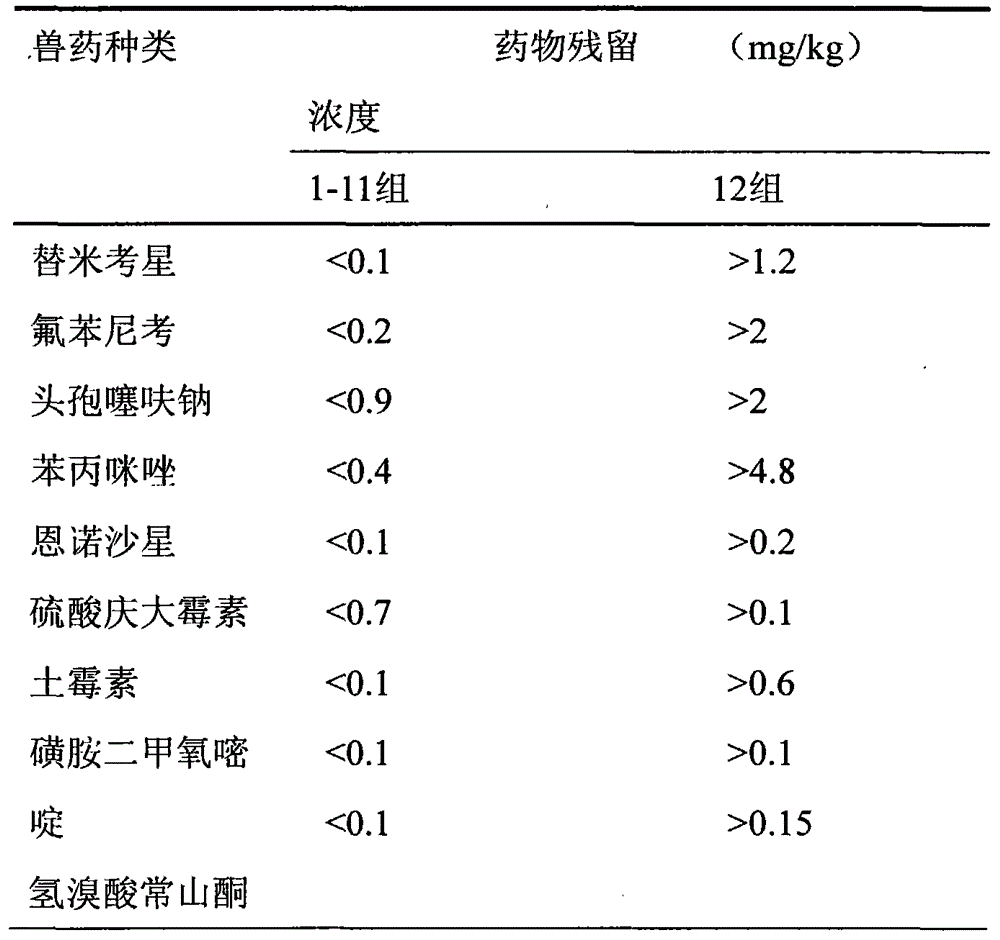
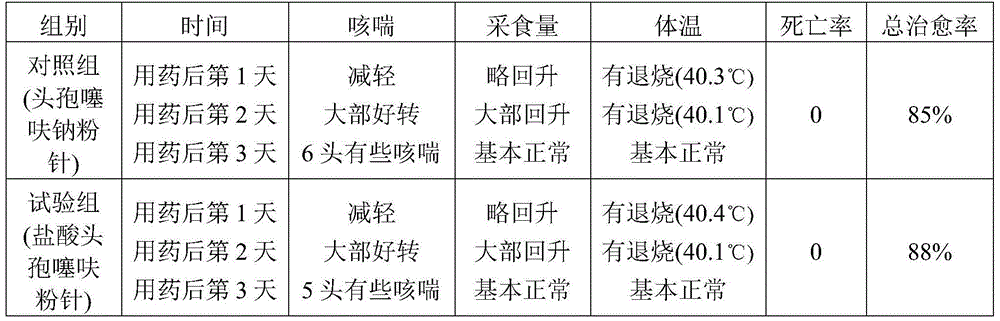
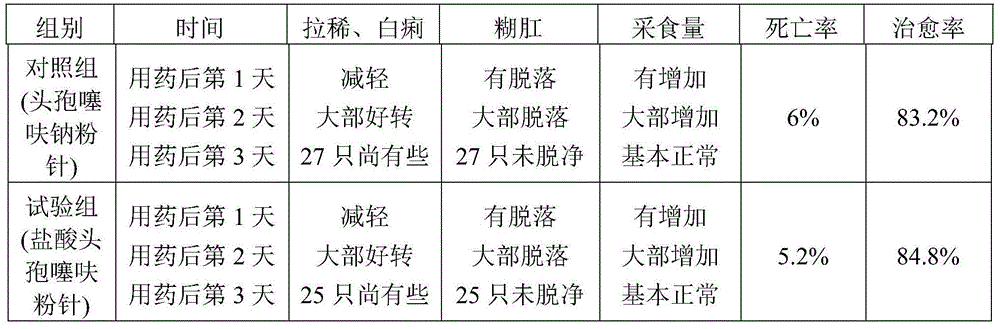
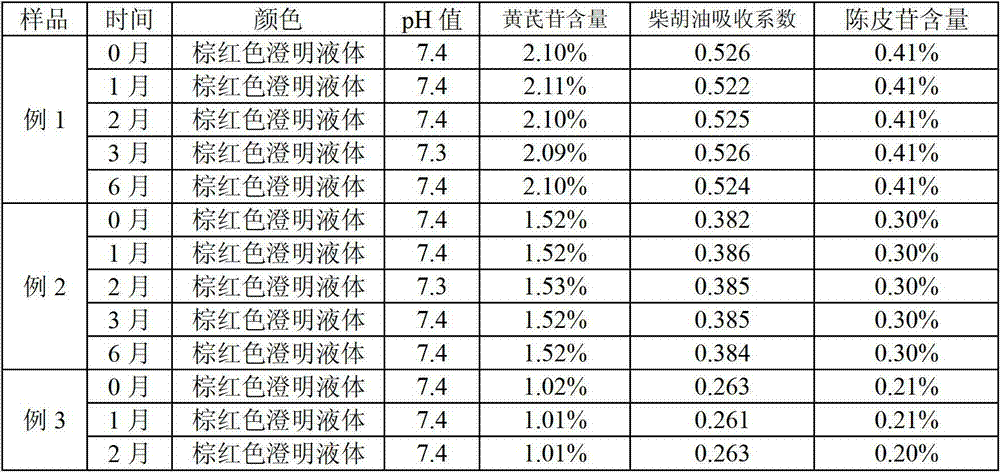
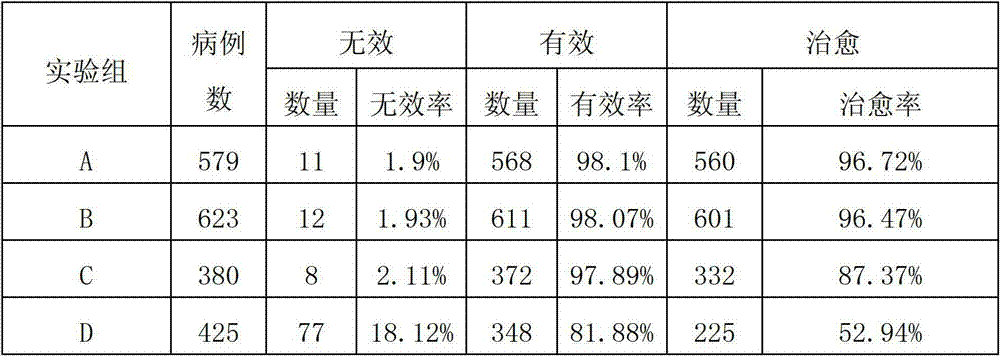
Ceftiofur sodium for Injection contains the sodium salt of ceftiofur which is a broad spectrum cephalosporin antibiotic active against Gram-positive and Gram-negative bacteria including β-lactamase-producing strains.
Chitosan nanoparticle preparation of ceftiofur sodium, and preparation method thereof
InactiveCN102319219AAchieve sustained releaseProlong the action timeAntibacterial agentsPowder deliveryChitosan nanoparticlesCeftiofur sodium
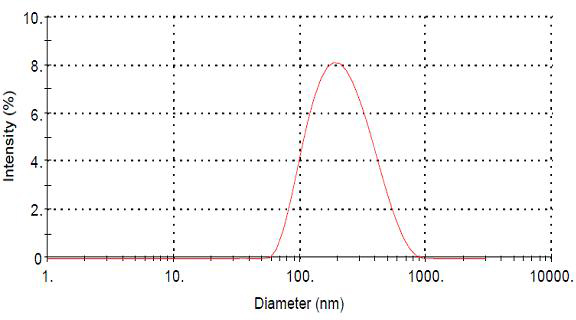
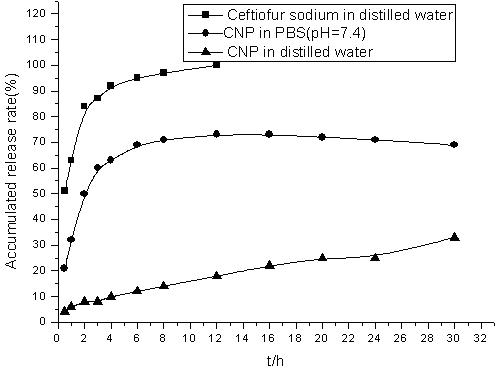
The invention belongs to the field of drug nanopreparation, especially relates to a chitosan nanoparticle preparation of ceftiofur sodium, and a preparation method thereof. The chitosan nanoparticle preparation is characterized in that: the preparation comprises 3-7 mg / mL of chitosan, 1.13-2.63 mg / mL of sodium tripolyphosphate and 2-5 mg / mL of ceftiofur sodium. The preparation method adopts an ionic crosslinking method and comprises the following steps: dissolving the chitosan, dissolving the ceftiofur sodium in the sodium tripolyphosphate solution, adding the sodium tripolyphosphate solution containing the ceftiofur sodium to the chitosan solution under magnetic stirring, and the like. According to the present invention, the ceftiofur sodium chitosan nanoparticles have target distribution and slow release property, such that the action time of the drug can be prolonged, the efficacy can be increased, the toxic and side-effect of the drug can be reduced, the antibacterial stability of the drug can be improved; the preparation method has characteristics of simpleness, reliability and controllable quality, and is suitable for the industrial production.
Owner:SICHUAN JINRUIKE ANIMAL PHARMA
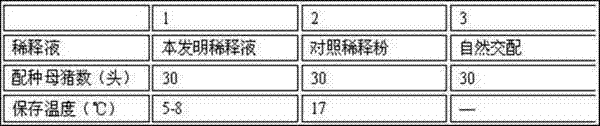
Boar antiviral long-acting semen diluent formula and preparation method
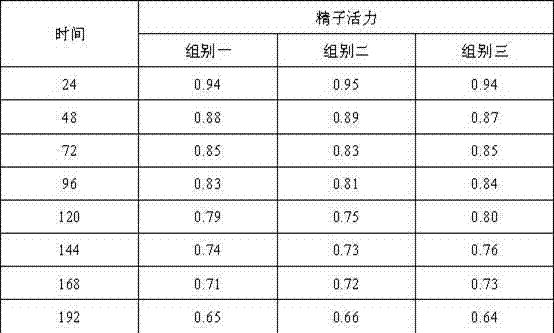
Belonging to the technical field of boar semen dilution, the invention relates to a boar antiviral long-acting semen diluent formula and a preparation method. The boar semen diluent comprises: alpha-D-glucopyranose powder, fructose, trisodium citrate dihydrate, ethylenediamine tetraacetic acid disodium, sodium bicarbonate, polyvinyl alcohol, trishydroxymethylaminomethane, inositol, vitamin C, arginine, a radix isatidis injection, a 5% ceftiofur sodium injection, and a 5% Ribavirin injection. The preparation method includes: dissolving all the components in distilled water, mixing the components evenly, and conducting sterilization to obtain the boar semen diluents. The formula involves rich nutrients, antibacterial Chinese and western medicine components and antibacterial components, thus ensuring normal sperm metabolism. After certain period of time at the lower limit of storage temperature, the effects of nutrition and sperm shock prevention can be realized, and the invasion of external bacterial viruses in an artificial insemination process can be avoided.
Owner:GUANGDONG ZHONGNONGLIAN BIOLOGICAL PHARMCO
Method for the preparation of ceftiofur sodium
InactiveUS6555680B1Avoiding product re-circulationAvoiding possible degradationOrganic chemistryCeftiofur sodiumOrganic chemistry
Owner:ORCHID CHEM & PHARM LTD
Compound preparation of ceftiofur sodium
InactiveCN101879172AAddressing drug resistanceImprove survival rateAntibacterial agentsHeterocyclic compound active ingredientsEscherichia coliCeftiofur sodium
The invention relates to a compound preparation of ceftiofur sodium, in particular to the application of a compound preparation of ceftiofur sodium in preparing medicines for treating colibacillus or salmonella infection of chicken and swine. The compound preparation of ceftiofur sodium comprises the following formula in parts by weight: 0.5-8 parts of ceftiofur sodium, 0.5-6 parts of fosfomycin sodium, 1-3 parts of sulbactam sodium and 4-7 parts of enorfloxacin sodium. The formula can be prepared into a premixed agent, a drink agent or an injection in the using process. The applicant of the invention, by summarizing of many years of tests and according to the antibacterial synergy principle, prepares the compound preparation of ceftiofur sodium which effectively solves the drug tolerance problem of the colibacillus and salmonella, simultaneously improves the action effect and the field of application and improves the survival rate of livestock and fowl.
Owner:杨建彬
Compound ceftiofur sodium freeze-dried power injection used for injection
ActiveCN102106857AHigh antibacterial activityDelay drug resistanceAntibacterial agentsPowder deliveryFLUNIXIN MEGLUMINECeftiofur sodium
The invention is a compound ceftiofur sodium freeze-dried power injection used for injection, and the compound ceftiofur sodium freeze-dried power injection provided by the invention is mainly composed of the following effective components in parts by weight: 2-4 parts of ceftiofur sodium, 1 parts of sulbactam sodium and 1 parts of flunixin meglumine; a vacuum freeze drying method is adopted to prepare the effective components into the compound ceftiofur sodium freeze-dried power injection used for injection. The compound ceftiofur sodium freeze-dried power injection has the advantages that the preparation cost can be lowered, and the drug resistance of bacteria on the ceftiofur sodium is relieved; and the broad antimicrobial spectrum is wide, the activity is strong, the drug administration times is less, and low stress and slight side effect are endured; and the negative effect of endotoxin can be effectively blocked.
Owner:QILU ANIMAL HEALTH PROD
Method for the preparation of ceftiofur sodium and its intermediates
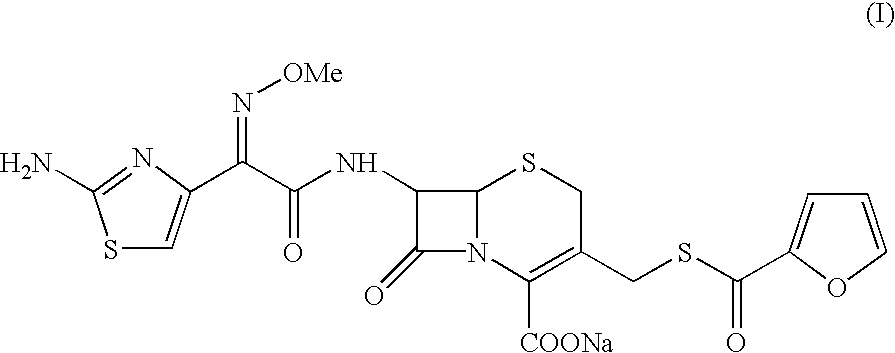
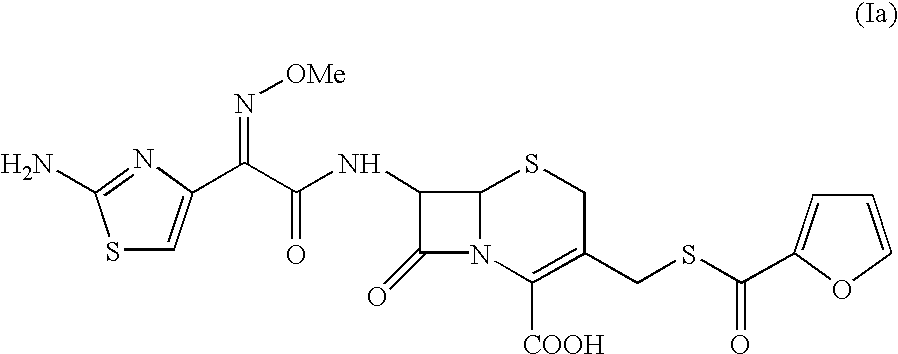
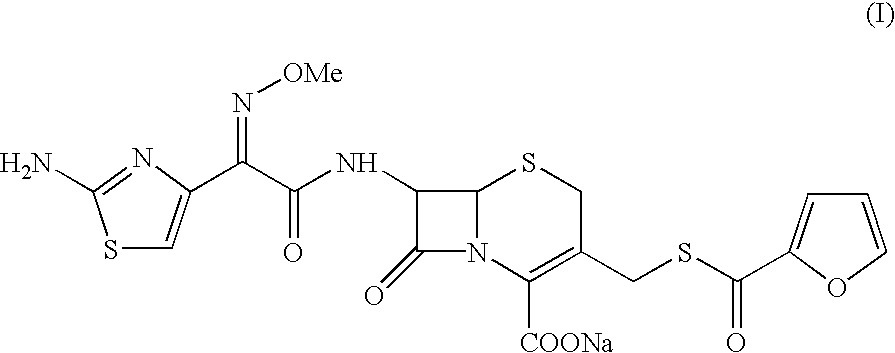
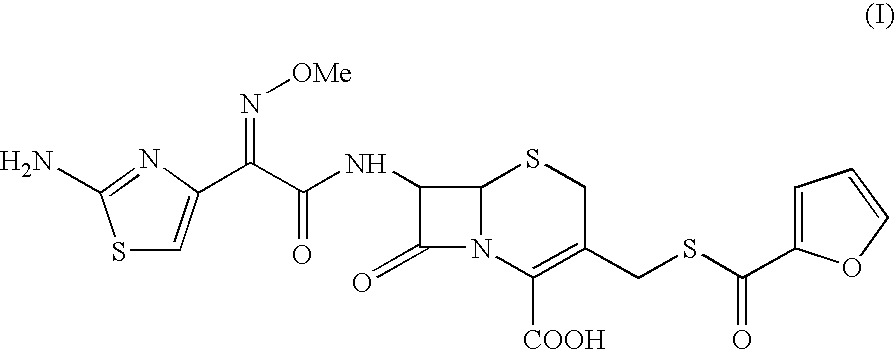
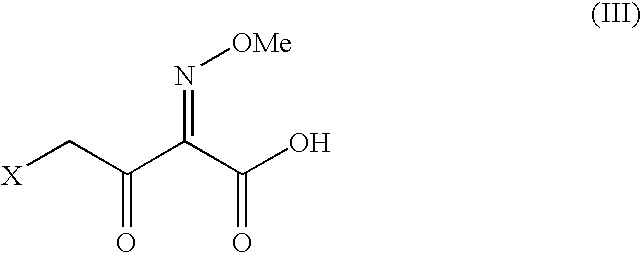
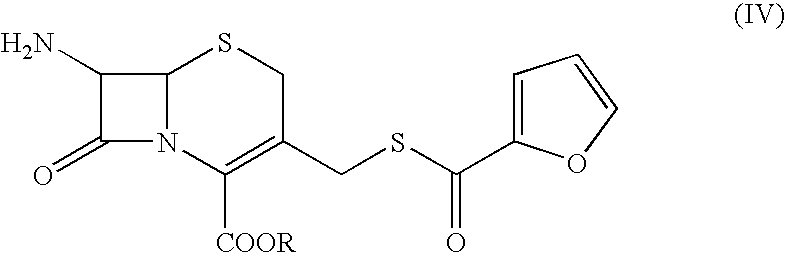
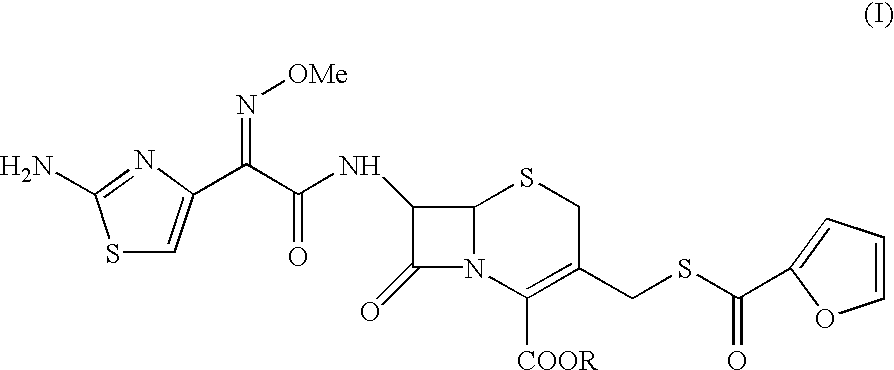
The present invention relates to preparation of Ceftiofur acid of formula (I),and its pharmaceutically acceptable salts. The process includes the steps of(i) condensing an activated derivative ofwherein the activated derivative is selected from acid halides, mixed anhydrides and active amides, and wherein X represents halogen atom selected from chlorine and bromine, with silylated derivative ofwherein R represents p-methoxybenzyl, p-nitrobenzyl or diphenylmethyl in the presence of a solvent at -40° C. to 0° C. to produce(ii) cyclising (V) with thiourea in the presence of water miscible solvent and sodium acetate at room temperature to produce cephalosporin(iii) deesterifying (VI) to produce (I) using anisole / trifluoroacetic acid, phenol / trifluoroacetic acid or formic acid at 0° C. to 10° C. and, if desired,(iv) converting (I), to its pharmaceutically acceptable salt. The invention also relates to intermediates (V) and (VI)
Owner:ORCHID CHEM & PHARM LTD
Natural compound feed additive for removing veterinary drug residues
InactiveCN104543404AAchieve productionGuarantee normal productionAnimal feeding stuffAstaxanthinVeterinary Drugs
The invention discloses a natural compound feed additive for removing veterinary drug residues. The natural compound feed additive is prepared from the following components in parts by weight: 1 part of procyanidine, 1 part of astaxanthin, 1 part of resveratrol, 1 part of curcumin, 1 part of allicin, 1 part of porphyra polysaccharide, 1 part of porphyra polyphenol, 1 part of phycobiliprotein, 1 part of tea polyphenol, 1 part of quercetin, 1 part of chitosan and 1 part of tangeretin; and the ratio of the feed additive to the feed is (1:100) to (1:500) in use. According to the method, the residues of tilmicosin, florfenicol, ceftiofur sodium, thiabendazole, enrofloxacin, gentamicin sulfate, oxytetracycline, sulfadimethoxine and halofuginone hydrobromide in livestock or poultry bodies can be effectively reduced; the natural compound feed additive has the characteristics of being free of pollution and free of public hazard, and can be applied to removal of veterinary drug residues from food animals of which the veterinary drug residues exceed the standard due the factors such as veterinary drug application and environmental pollution, so as to ensure the hygiene and safety of animal food.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Medicine for treating contagious ecthyma
InactiveCN102440997AInhibition of replicationAvoid infectionOrganic active ingredientsDigestive systemLamb mortalityTreatment effect
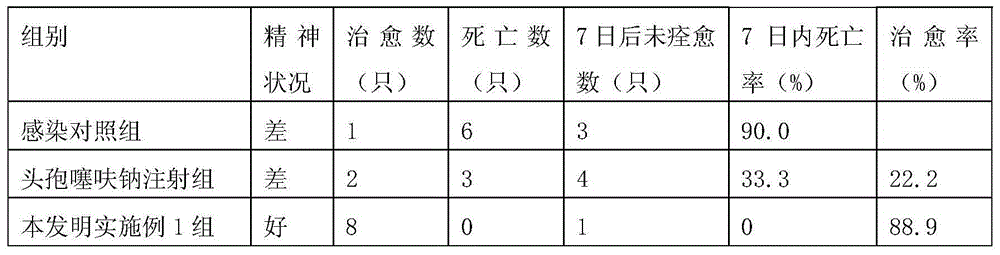
The invention discloses a medicine for treating contagious ecthyma. Each milliliter of the medicine comprises: 0.025-0.028g of ribavirin, 0.0035-0.004g of ceftiofur sodium, glycerin with a volume concentration of 2%-2.5%, and the balance normal saline. The medicine of the invention can rapidly inhibit replication of a contagious ecthyma virus, and prevent an aphthous ulcer surface from developing into secondary bacterial infections. The medicine provided in the invention has the advantages of uneasy volatilization, sufficient sources of medicine components, low price, convenient use, good treatment effect, cure rate up to 75%, lamb mortality declined by 5%, condition improvement rate of 90%, and no harm to breeders.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
New preparation method of ceftiofur sodium
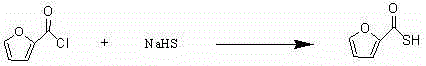
InactiveCN104530085AFew synthetic stepsSimplify operating proceduresOrganic chemistrySodium hydrosulfideCarboxylic acid
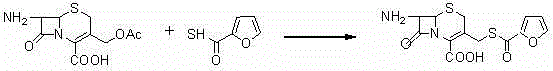
The invention discloses a new preparation method of ceftiofur sodium. The method comprises the following steps: synthesizing 2-furylcarbothiolic acid and sodium hydrosulfide, synthesizing 7-amino-3-[(2-furyl-carbonyl)-sulfomethyl]-3-cephem-4-carboxylic acid from 7-ACA and 2-furylcarbothiolic acid in a boron trifluoride ether complex and acetonitrile, reacting 7-amino-3-[(2-furyl-carbonyl)-sulfomethyl]-3-cephem-4-carboxylic acid with AE-active ester, and directly reacting the obtained substance with sodium iso-octoate to obtain ceftiofur sodium. The method shortens synthesis step and simplifies the operating process, so the method has the advantages of cheap and easily available raw materials and reagents, simple operation, environmental protection, high yield, low cost, and suitableness for industrial production.
Owner:HENAN LINGXIAN SCI & TECHN PHARMA
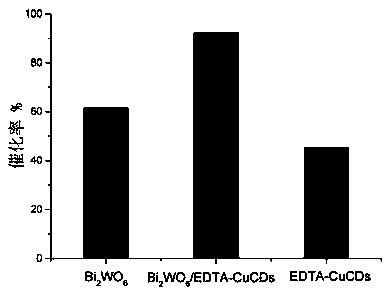
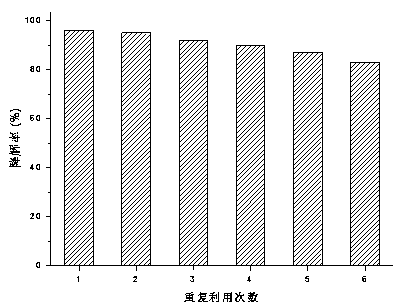
Preparation method and application of copper-doped CQD (carbon quantum dot)/bismuth tungstate composite photocatalyst
ActiveCN108786787AEasy transferHigh catalytic activityWater/sewage treatment by irradiationWater contaminantsCeftiofur sodiumElectron donor
Owner:KUNMING UNIV OF SCI & TECH
Drug for enhancing immunity of animals
InactiveCN103169762AReduce manufacturing costImprove stabilityAntibacterial agentsOrganic active ingredientsDiseaseMongolian oak
The invention discloses a drug for enhancing the immunity of animals. The drug comprises the following components by weight percent: 40-50% of echinacea, 10-15% of herba epimedii, 10-20% of gensing, 20-30% of bee glue, 10-15% of mongolian oak peel, 10-15% of liquorice, 5-10% of honeysuckle and 5-10% of ceftiofur sodium. The drug can be independently and orally taken or used as a feed additive and is low in production cost, good in stability and suitable for industrial production. Proved by clinical experiments, the drug can be used for effectively enhancing the immunity of a human body, remarkably reducing various bacteria and the morbidity of viral diseases and being matched with other drugs to treat various animal diseases. The invention also discloses a preparation method of an oral liquid of the drug for enhancing the immunity of the animals.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD +1
Method for preparation of ceftiofur sodium from its hydrohalide salts
The present invention relates to a process for preparing sodium salt of cephalosporins from their corresponding hydrohalide salt, which is neutralized with trimethylsilylating agent for the first time.
Owner:ORCHID CHEM & PHARM LTD
Ceftiofur hydrochloride powder injection as well as preparation method and application thereof
ActiveCN104586777AProlong the action time of concentrationWell mixedAntibacterial agentsPowder deliveryCefepime hydrochlorideFreeze-drying
The invention belongs to the technical field of veterinary medicines, and particularly relates to a ceftiofur hydrochloride powder injection as well as a preparation method and an application thereof. The ceftiofur hydrochloride powder injection is prepared from the following effective components ceftiofur hydrochloride and a cosolvent at the weight ratio of (48-52) to (28-34). Ceftiofur sodium is replaced with the ceftiofur hydrochloride; veterinary cefepime hydrochloride powder injection is prepared by weighing the effective components and the cosolvent at the weight ratio of (48-52) to (28-34), mixing evenly, and sub-packaging; and the veterinary cefepime hydrochloride powder injection can be produced and stored at a room temperature. According to the ceftiofur hydrochloride powder injection, the problems that an existing veterinary cefepime hydrochloride powder injection needs to be produced by a freeze-drying method, the raw materials and the preparation need to be stored at a low temperature of 4-10 DEG C, the placement time in a room-temperature or high-temperature season is overlong, and the medication effect is poor due to degradation are solved; the prepared powder injection has the same antibacterial effect as a ceftiofur sodium freeze-dried powder injection; and the ceftiofur hydrochloride powder injection is low in cost and wide in application prospect.
Owner:广东省天宝生物制药有限公司
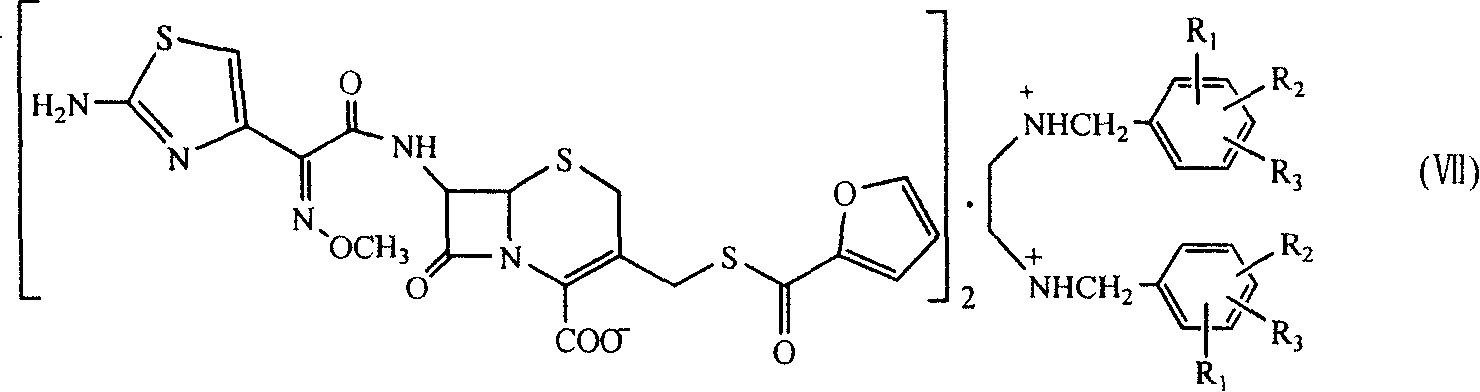
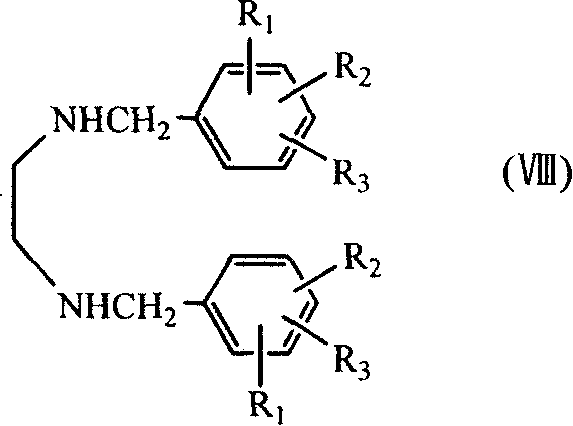
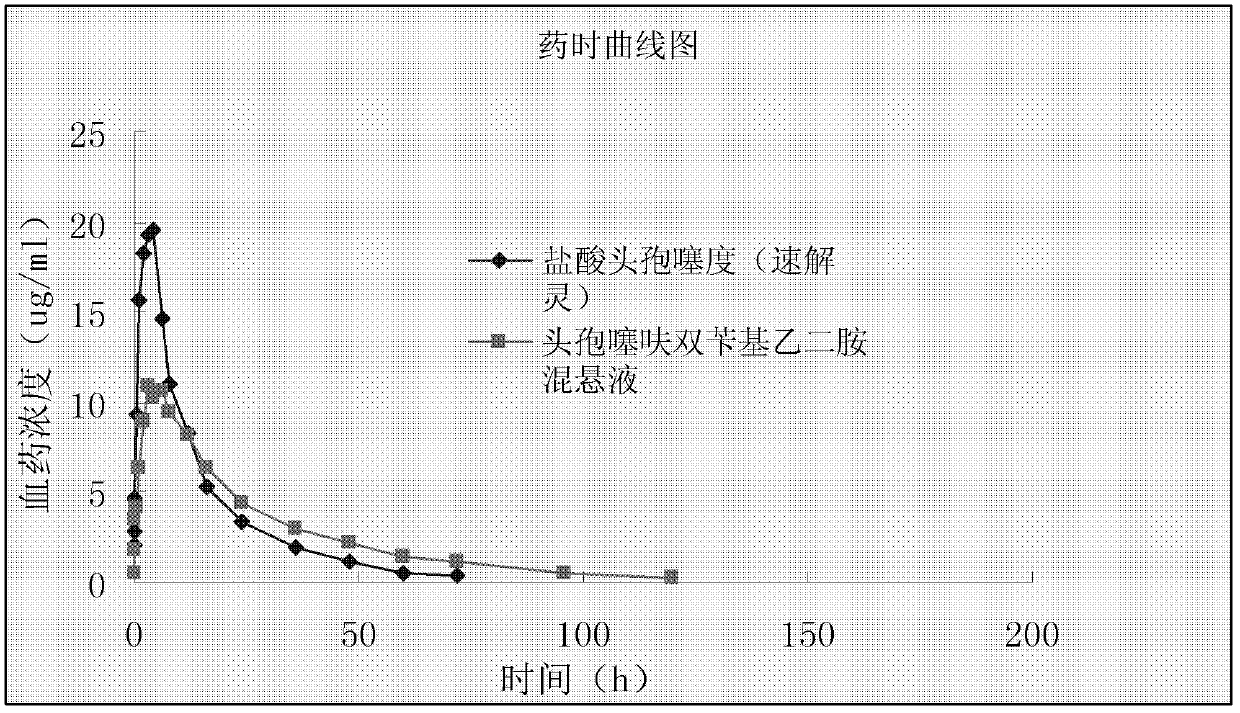
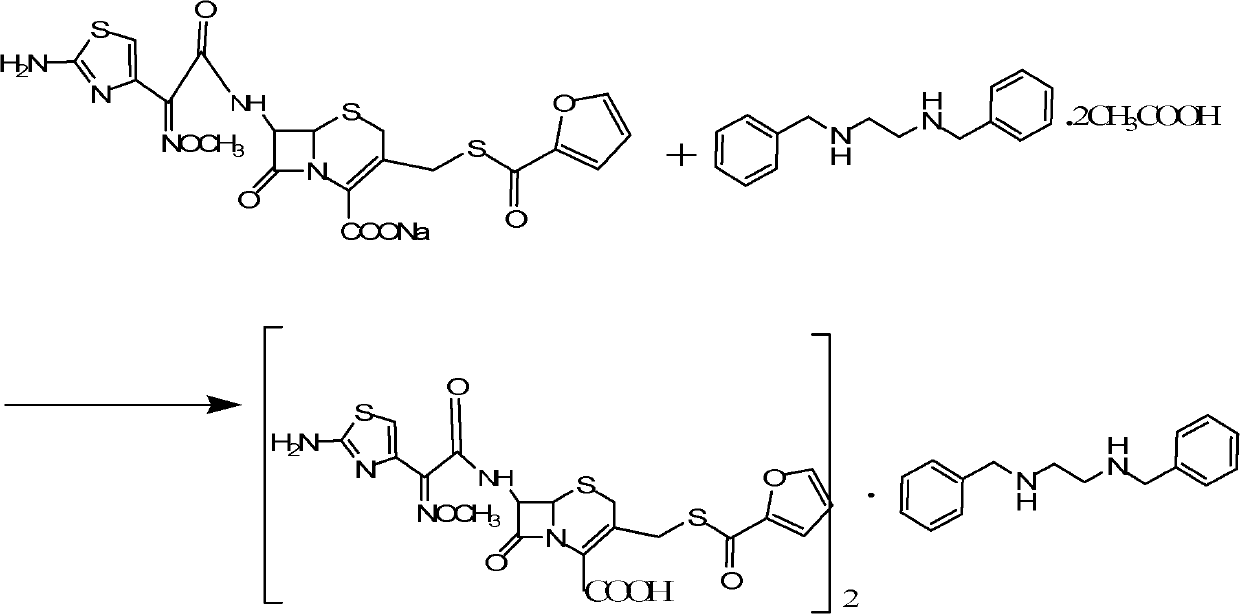
Benzathine salt of ceftiofur, preparation method and application thereof
ActiveCN101245078AAvoid degradationReduce solubilityOrganic chemistryEthylenediamineCeftiofur sodium
The invention relates to the double benzyl (or the substituted benzyl) ethylenediamine salt of Ceftiofur, the preparation method and the application in the preparation of ceftiofur sodium salt. The double benzyl (or the substituted benzyl) ethylenediamine salt of Ceftiofur is adopted for the preparation of ceftiofur sodium by the treatment of cation resin, the preparation process and the processing are simple, the purity of products is high and the technique is stable, thus being suitable for the industrial production.
Owner:QILU ANIMAL HEALTH PROD +1
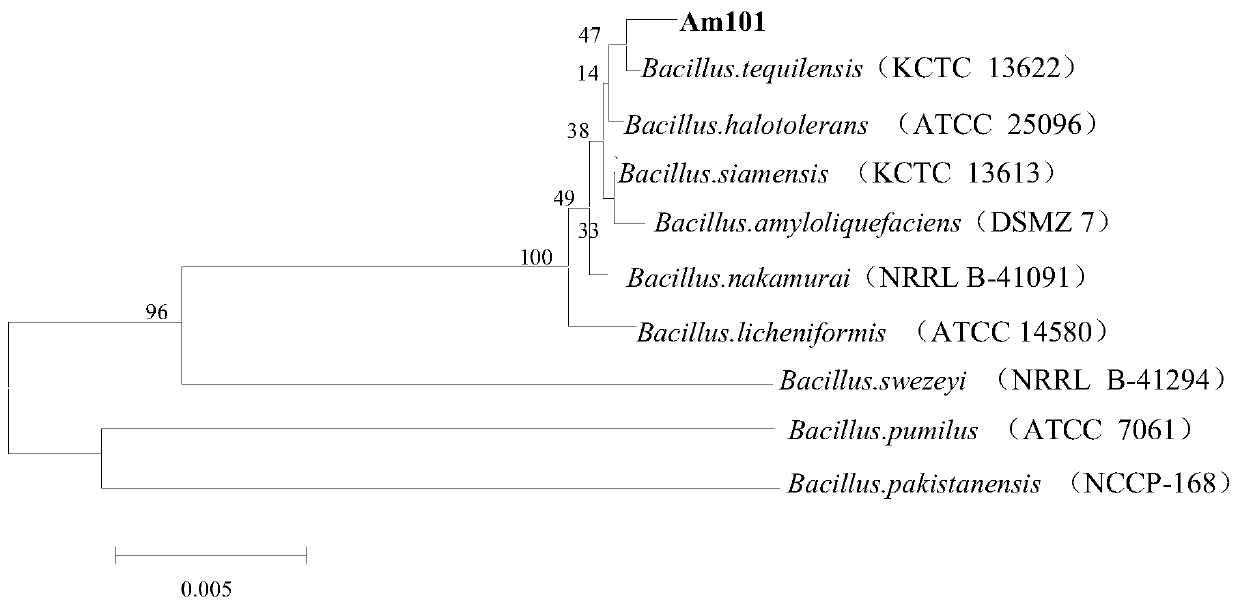
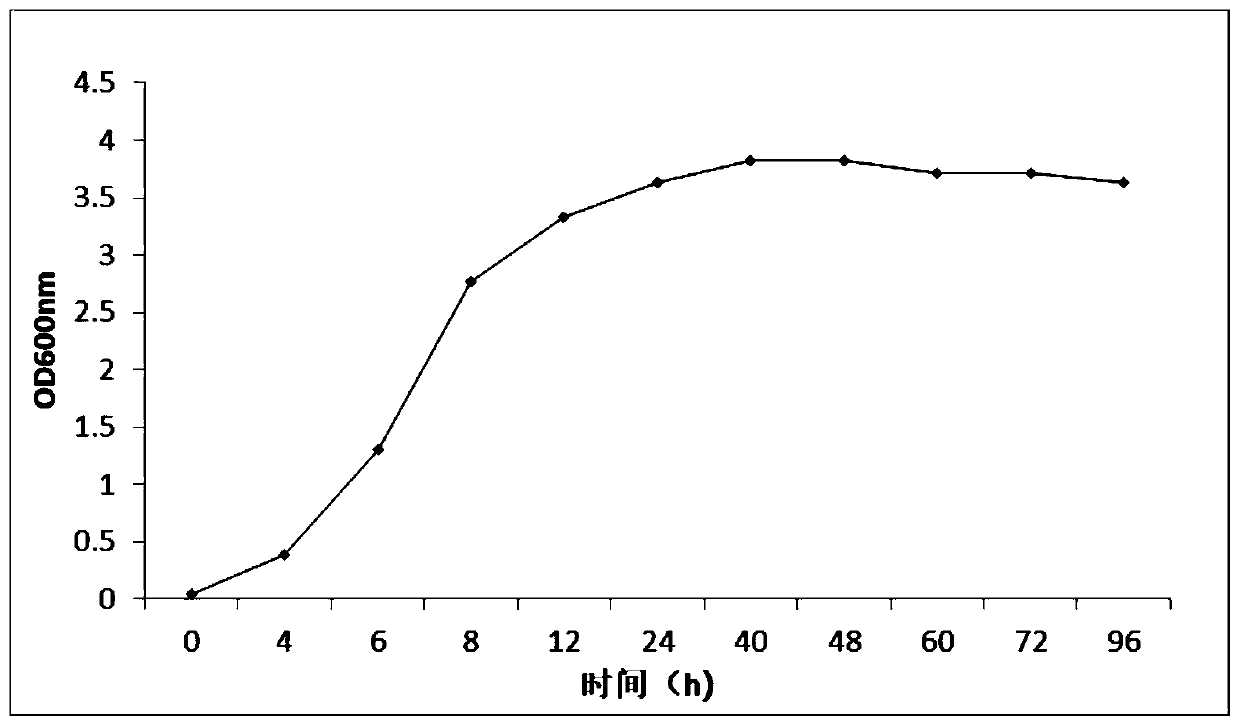
Strain Am101 capable of degrading various beta-lactam antibiotics and application of strain Am101
ActiveCN110791450ABroad degradation spectrumFast degradationBacteriaWater contaminantsBiotechnologyBenzylpenicillin potassium
The invention discloses bacillus tequilensis Am101 capable of degrading various beta-lactam antibiotics and an application of the bacillus tequilensis Am101. The strain is preserved in China General Microbiological Culture Collection Center on November 18, 2019, and a preservation number is CGMCC NO. 18965. The strain Am101 disclosed by the invention can be used for simultaneously degrading various beta-lactam antibiotics and can be used for efficiently degrading benzylpenicillin potassium, amoxicillin, cefuroxime sodium and ceftiofur sodium. The strain Am101 can be applied to degradation treatment of residual beta-lactam antibiotics in livestock and poultry manure, can also be applied to restoration of water and soil polluted by the beta-lactam antibiotics, and has the wide application prospect.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
New preparation method of ceftiofur sodium
The invention provides a new method for preparing sodium salt from ceftiofur hydrochloride. The method comprises the following step of reacting inorganic weak base such as sodium bicarbonate and the like or organic weak acid with the ceftiofur hydrochloride by using a proper solvent to obtain the sodium salt with high yield. The quality of products reaches quality standard of the Ministry of agriculture. Compared with the conventional process route, the process route has the characteristics of cheap and readily-available selected raw materials and reagents, low process cost, environmental friendliness, high product yield (about 90 percent) and the like and is suitable for industrialized production.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
Medicinal composition containing ceftiofur bisbenzylethylenediamine
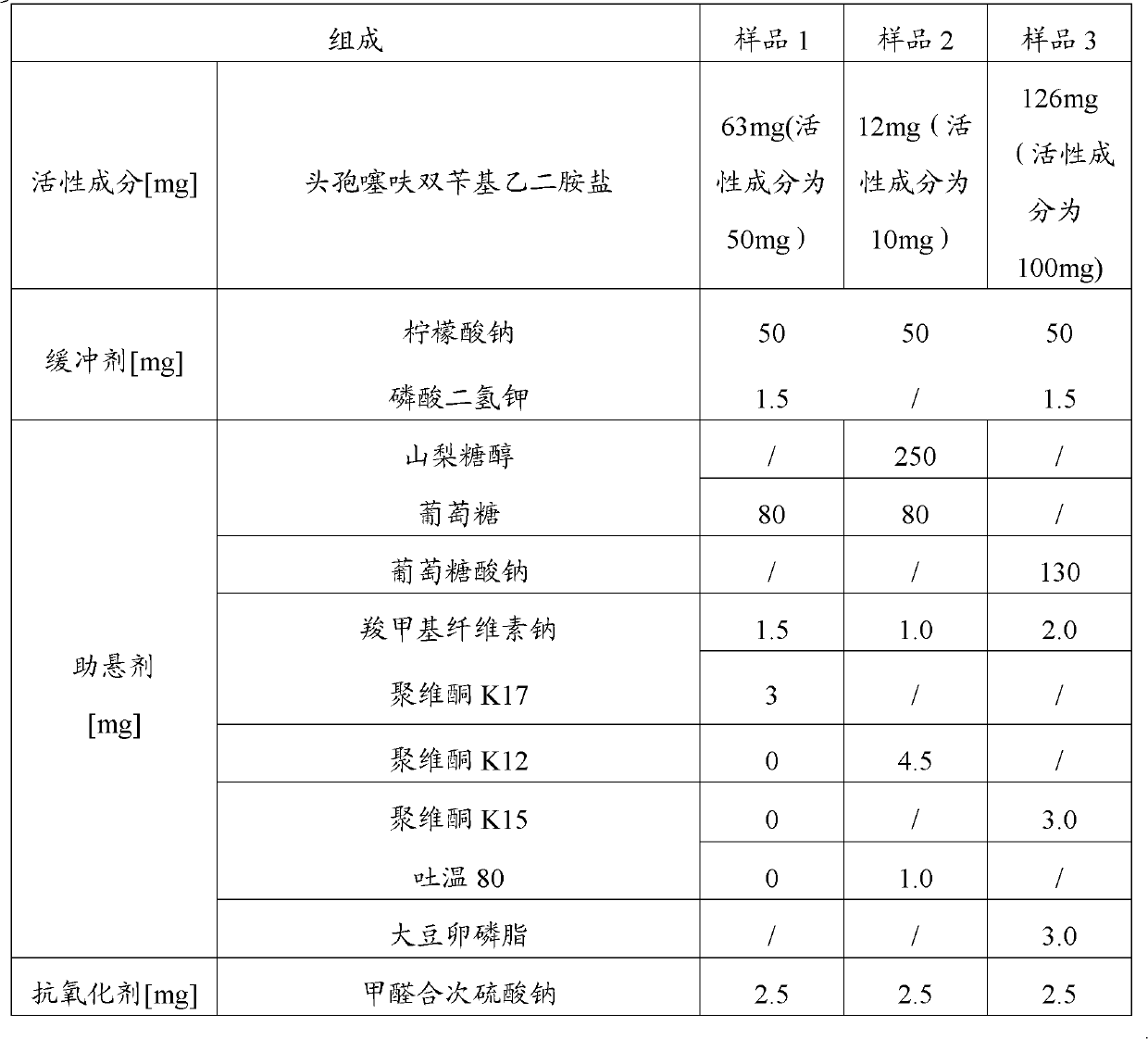
InactiveCN102885820ALow costImprove stabilityAntibacterial agentsOrganic active ingredientsBacterial respiratory infectionCeftiofur sodium
The invention provides a suspension injection of ceftiofur bisbenzylethylenediamine, which comprises the following components: 1.2% to 12.6% (w / v) of superfine powdery ceftiofur bisbenzylethylenediamine and 0.1% to 25% (w / v) of buffering agent, and the invention also provides the preparation steps of the suspension injection of ceftiofur bisbenzylethylenediamine. The invention discloses the suspension injection of ceftiofur bisbenzylethylenediamine and the medicinal value of the suspension injection of ceftiofur bisbenzylethylenediamine, namely at least the efficacy of ceftiofur is achieved. The suspension injection of ceftiofur bisbenzylethylenediamine provided by the invention can be prepared into the easily absorbed aqueous liquid dosage forms for treating bacterial respiratory infection of livestock, such as the preparation and oral liquid. Compared with the prior art, the suspension injection has the advantages that at least a step of preparing ceftiofur sodium by ceftiofur bisbenzylethylenediamine is saved, and the problems that the preparation steps of ceftiofur sodium are complicated and the cost is high are avoided.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Method for preparation of ceftiofur sodium from its hydrohalide salts
The present invention relates to a process for preparing sodium salt of cephalosporins from their corresponding hydrohalide salt, which is neutralized with trimethylsilylating agent for the first time.
Owner:ORCHID CHEM & PHARM LTD
Compound ceftiofur sodium injection
ActiveCN104352501AImprove stabilityHigh antibacterial activityAntibacterial agentsHydroxy compound active ingredientsGlycineEscherichia coli
The invention provides a compound ceftiofur sodium injection with significant antibacterial action. The compound ceftiofur sodium injection comprises the following components in parts by weight: 1-4 parts of ceftiofur sodium, 1-2 parts of magnolol, 0.5-1 part of sodium sulfite, 0.5-1 part of glycine and 1-2 parts of Tween. The injection disclosed by the invention is high in stability and simple in preparation process, has a good inhibiting effect on swine escherichia coli, has an accurate curative effect on porcine colibacillosis, is capable of reducing the dosage of a prescribed preparation and shortening the treatment cycle, and has a wide market prospect.
Owner:CHONGQING TAITONG ANIMAL PHARMA
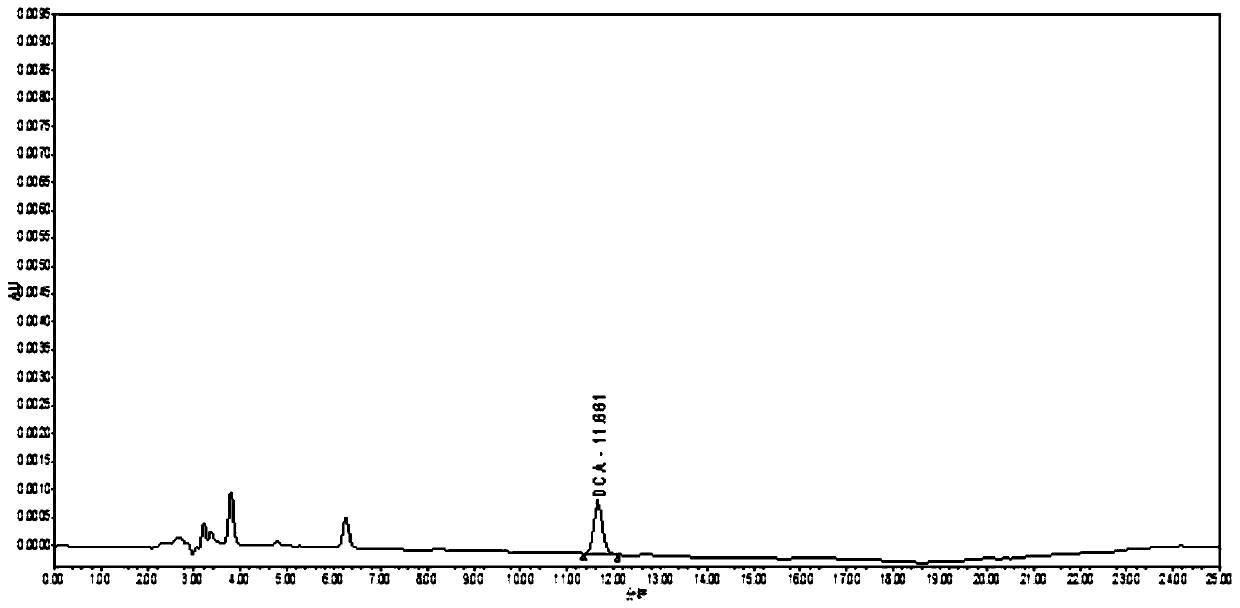
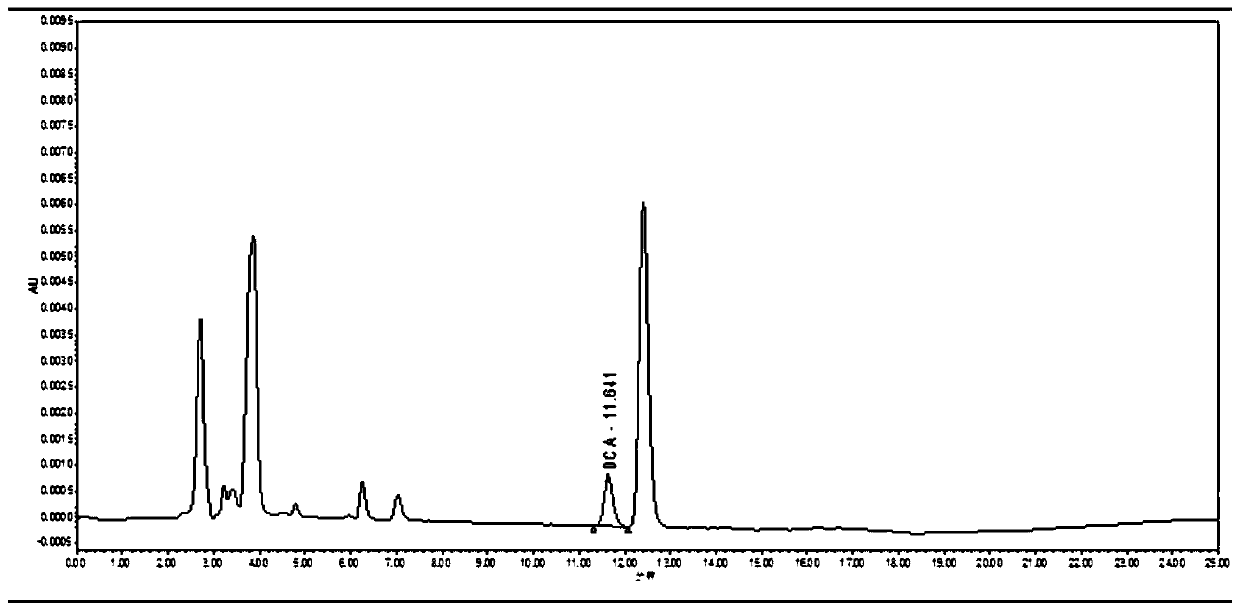
Pharmacokinetics and elimination detection method of sodium ceftiofur in chicken body
InactiveCN110412147AEnabling Pharmacokinetic StudiesComponent separationCeftiofur sodiumBlood plasma
The invention discloses a pharmacokinetics and elimination detection method of sodium ceftiofur in chicken body. The method comprises the following steps: for the pharmacokinetics characteristics of four sodium ceftiofur products for injection in the yellow chicken body in one-day old, selecting a health chick in the one-day old for each medicine, subcutaneously injecting 0.1mg of sodium ceftiofurinto each chick, detecting desfuroyl ceffiofur (DFC) concentration in plasma through a high-performance liquid chromatography, researching the pharmacokinetics of the DFC in the yellow chicken body in one-day old, thereby realizing the research of the pharmacokinetics of the sodium ceftiofur in the yellow chicken body in one-day old.
Owner:WENS FOOD GRP CO LTD +1
Composition and methods of treatment of bacterial meningitis
ActiveUS20120022031A1Avoiding massive dosMinimize undesirable reactionAntibacterial agentsBiocideCeftiofur sodiumIntensive care medicine
A diluted solution of ceftiofur sodium is intrathecally or ventricularly delivered to effectively treat bacterial meningitis while maintaining the patient's threshold and reducing the likelihood of seizure.
Owner:NBR PATHFINDER
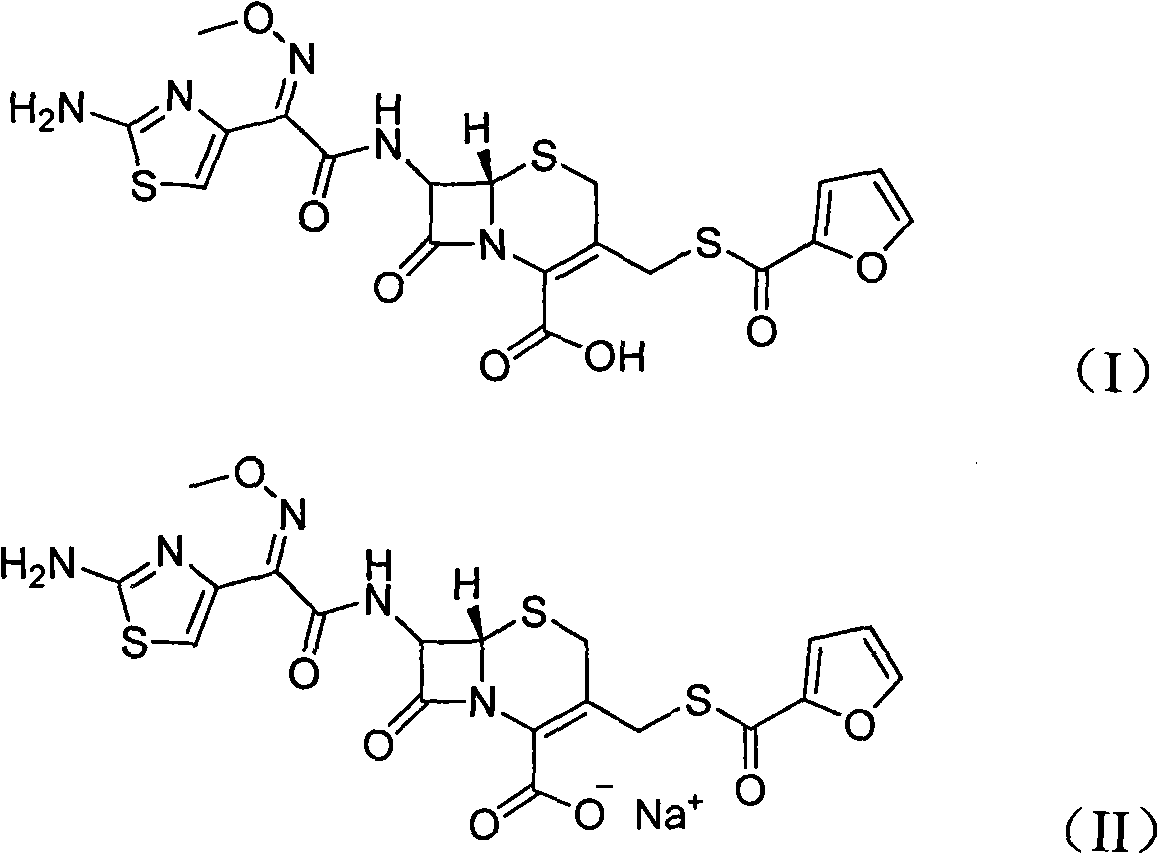
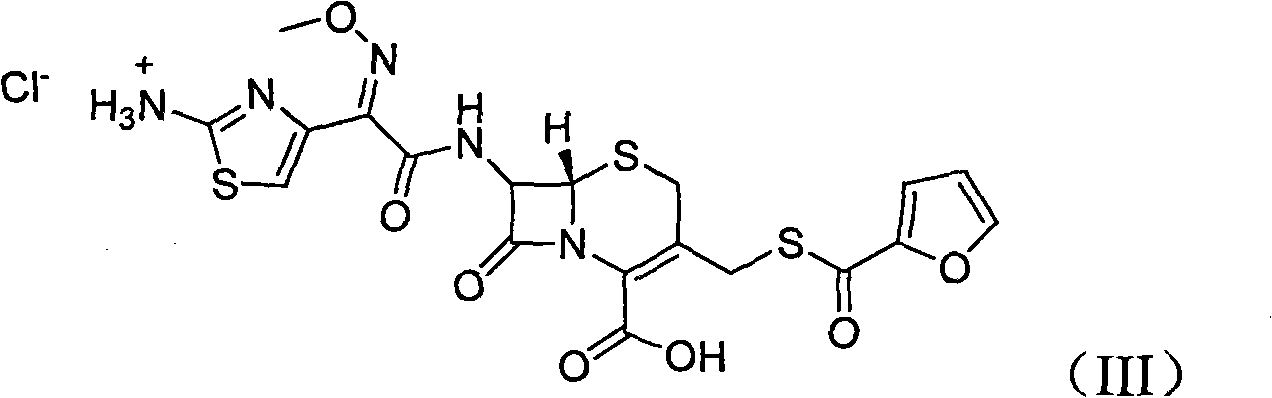
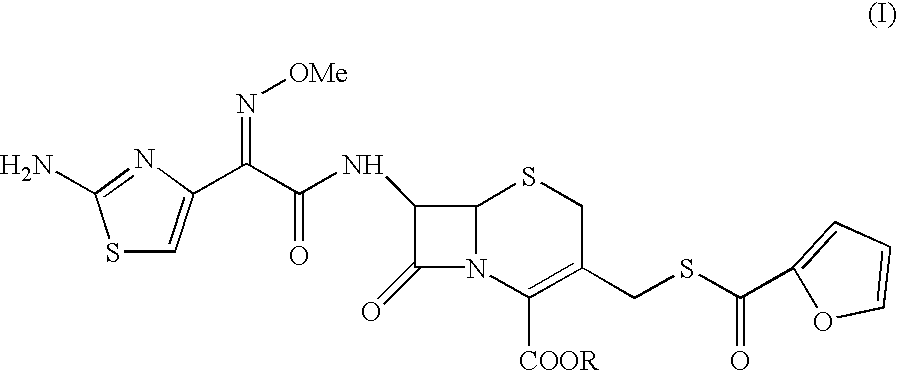
Method for preparation of ceftiofur and salts thereof
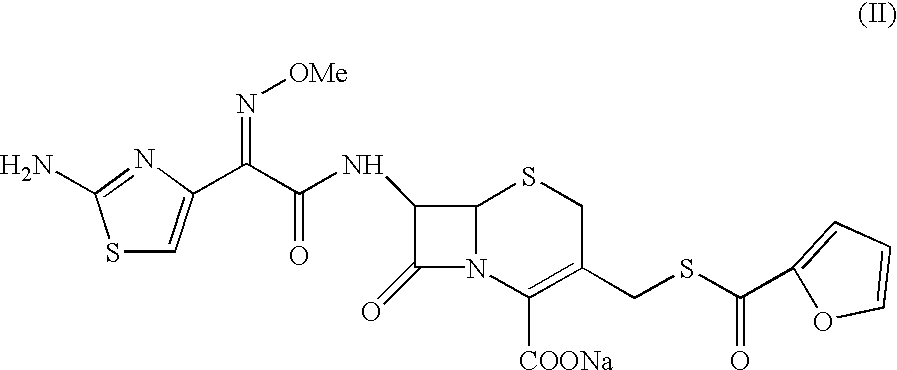
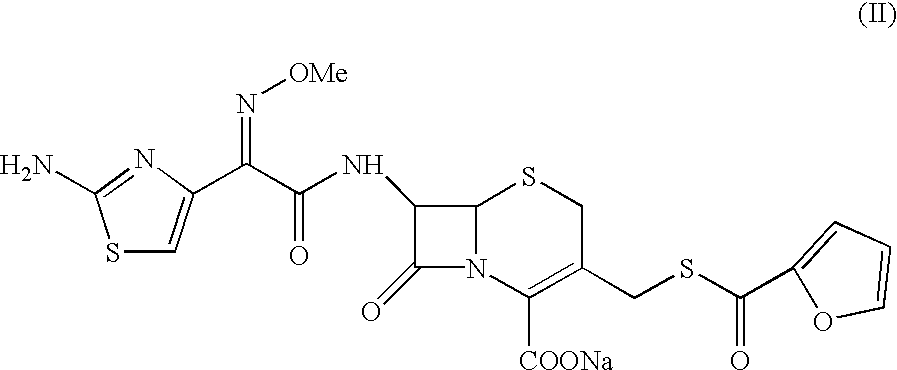
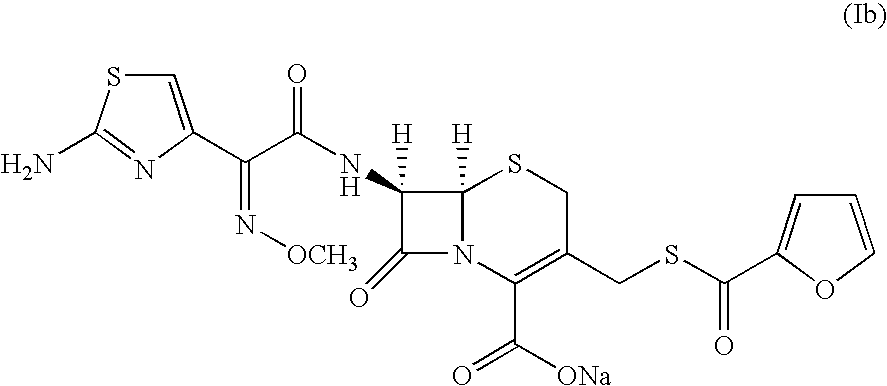
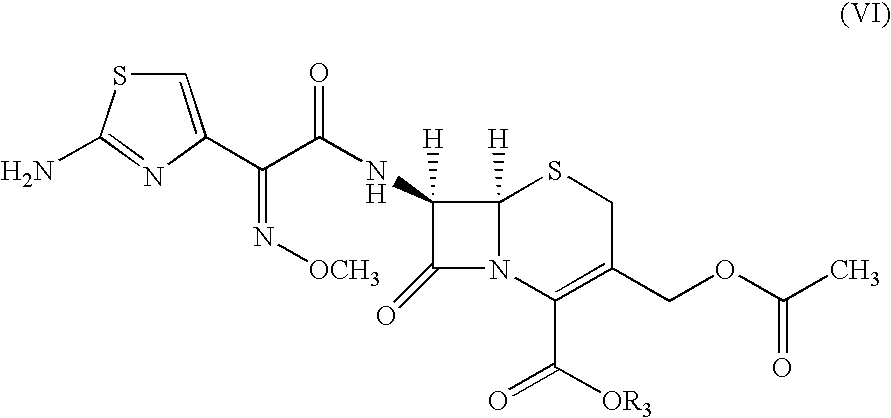
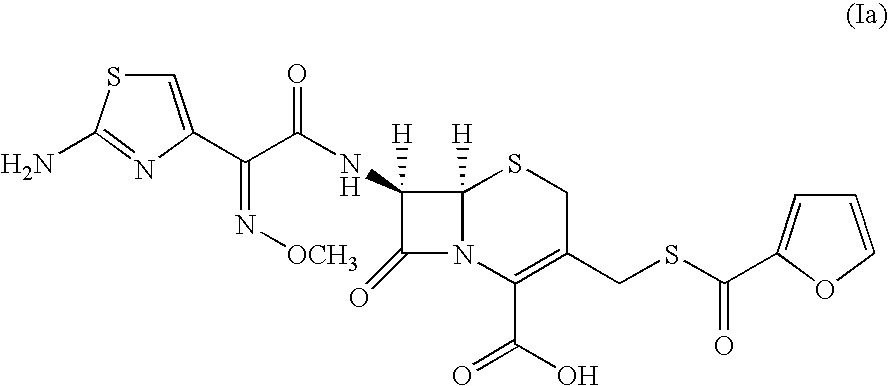
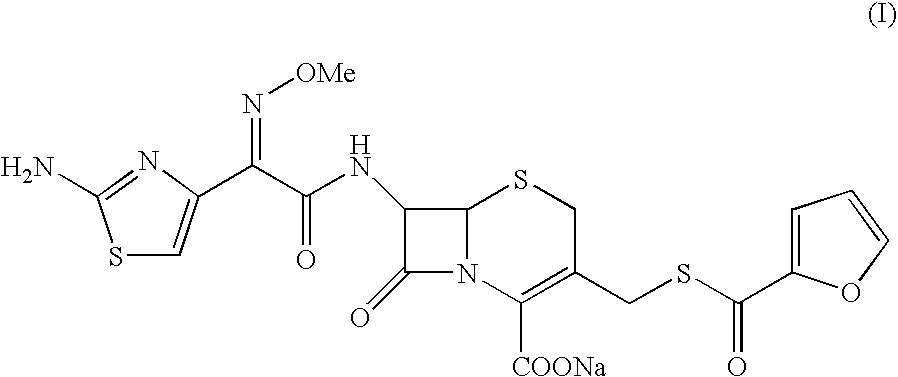
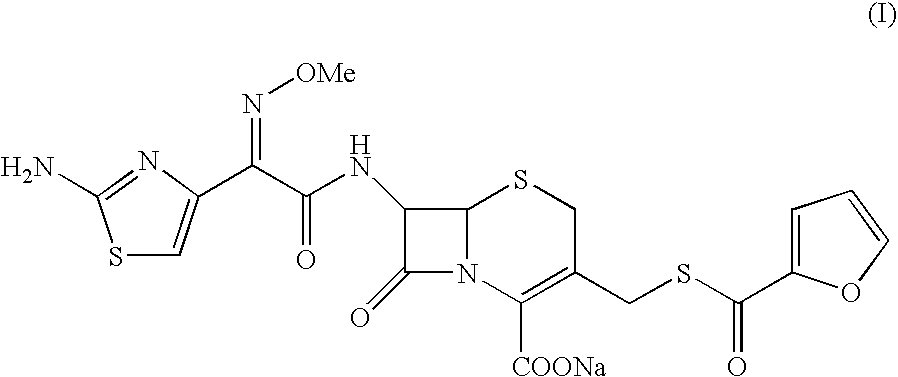
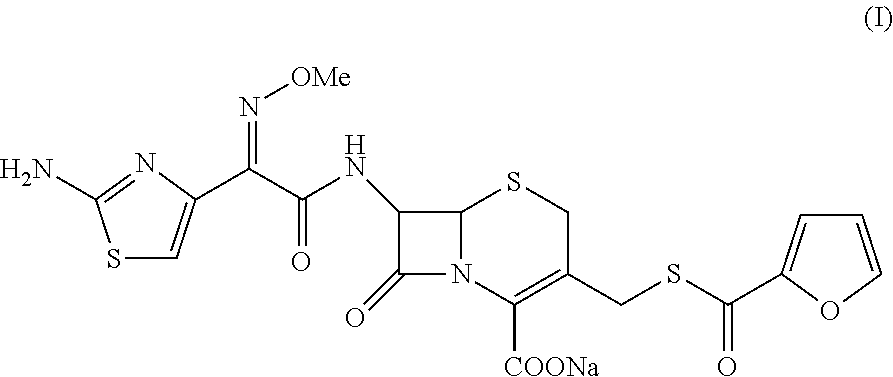
A process for preparation of ceftiofur sodium of formula (Ib)possessing high stability and having purity of more than 97% and substantially free of impurities, is disclosed. The process comprises:i) reacting cefotaxime or its salts or its esters of formula (VI)wherein R3 is hydrogen, an alkali or alkaline earth metal, or an easily hydrolysable ester, with thiofuroic acid, employed in a molar proportion of 1.5 to 3.0 moles per mole of compound (VI), in the presence of acetonitrile as solvent and in the presence of large excess of methanesulfonic acid, employed in molar proportions of 12 to 18 moles per mole of compound (VI), and at a temperature of between −5° C. to 30° C. to give after necessary neutralization of the alkali or alkaline earth metal or removal of the ester group of the 4-carboxylic acid function, wherever applicable, ceftiofur of formula (Ia), possessing high stability and having purity of more than 97% and substantially free of impurities;ii) converting the ceftiofur of formula (Ia) thus obtained to its salt with an organic amine by treating a solution of ceftiofur in a mixture of water and a water-miscible organic solvent with an organic amine, at a temperature ranging from −10° C. to 10° C.;iii) reacting of the amine salt thus obtained with a sodium metal carrier in a mixture of water and water-miscible organic solvent and in presence of sodium hydrogen sulfite to give ceftiofur sodium of formula (Ib).
Owner:LUPIN LTD
Composition for treating piglet staphylococcal exudative epidermitis
ActiveCN104147044AAvoid distribution throughout the body,Avoid the disadvantage of difficult to reach the lesion siteAntibacterial agentsInorganic active ingredientsVitamin CCuticle
The invention discloses a composition for treating piglet staphylococcal exudative epidermitis. The composition comprises 0.1-2% by mass of mometasone furoate, 79.9-93% by mass of a mixed reagent including ceftiofur sodium, vitamin C and zinc oxide, and the balanced a suspending agent of a dry suspension. The disclosed external-use medicine composition is used for treating exudative epidermitis caused by piglet staphylococcus. The ceftiofur sodium can quickly kill the staphylococcus; the mometasone furoate can effectively inhibit irritability, dermatitis and metabolic disorder caused by skin local immune disorder caused by fallen-off toxins; and the vitamin C and the zinc oxide can quickly induce skin to regenerate. Compared with conventional antibiotic injection treatment, a preparation of the composition can greatly significantly increase a curative ratio of the piglet staphylococcal exudative epidermitis. In addition, piglets can get well quicker. The composition has a wide application prospection.
Owner:HUNAN AGRICULTURAL UNIV
Medicine formula for eliminating in-vivo enolotoxin
ActiveCN104800220AEffective treatmentInhibition of reproductionAntibacterial agentsAntipyreticEscherichia coliFLUNIXIN MEGLUMINE
The invention relates to a medicine formula for eliminating in-vivo enolotoxin. The medicine formula comprises a formula A and a formula B, wherein the formula A comprises raw materials in parts by weight as follows: 1-4 parts of flunixin meglumine and 1-6 parts of vitamin C; the formula B comprises raw materials in parts by weight as follows: 2-8 parts of ceftiofur sodium and 1.5-5 parts of enrofloxacin, wherein flunixin meglumine is an injection with a specification of 0.05g / ml, the vitamin C is an injection with a specification of 0.1g / ml, enrofloxacin is an injection with a specification of 0.05g / ml, and ceftiofur sodium is a freeze-dried powder injection. The medicine formula can effectively eliminate enolotoxin causing diseases to animals, has high sensitivity to pathogenic bacteria such as escherichia coli, infectious actinobacillus pleuropneumoniae, haemophilus parasuis and the like, can be used for effectively treating diseases such as hydropsy, infectious pleuropneumonia, fevering, loss of appetite and the like caused by the pathogenic bacteria and has remarkable curative effects.
Owner:SICHUAN CHENGKANG ANIMAL PHARMA
Immunostimulatory injection for livestock
InactiveCN102727631AImprove growthVarious ways to useAntibacterial agentsAntipyreticBENZYL ALCOHOL/WATERInjection solution
The invention relates to an immunostimulatory injection for livestock. The injection can be used as a diluent to enhance immune effects of vaccine especially serum and strongly enhance the antibacterial efficacy of the antibiotic powder injection; and the injection can be individually used to protect liver, promote bile flow, bring down a fever and improve the growth performance of animal bodies. The invention is characterized in that immunostimulatory injection is prepared by the following steps of: taking 10-20 g of Radix Astragali and 5-10 g of dried orange peel, slicing, decocting, concentrating by the use of ethanol to prepare a liquid A for standby; cutting off 5-10 g of radix bupleuri, adding water and distilling, dissolving by adding PEG 400 so as to prepare a liquid B for standby; adding the liquid B into the liquid A, adding injection water to 85-95 ml, using a pH conditioning agent, adding 0.1-0.2 g of active carbon, slightly boiling for 1 h, standing until it cools, adding 1 ml of benzyl alcohol for a constant volume of 100 ml; filtering, embedding, and sterilizing. The injection is produced at low cost, has good effect when singly used or used as a diluent, and especially has substantial synergism for ceftiofur sodium.
Owner:金河牧星(重庆)生物科技有限公司
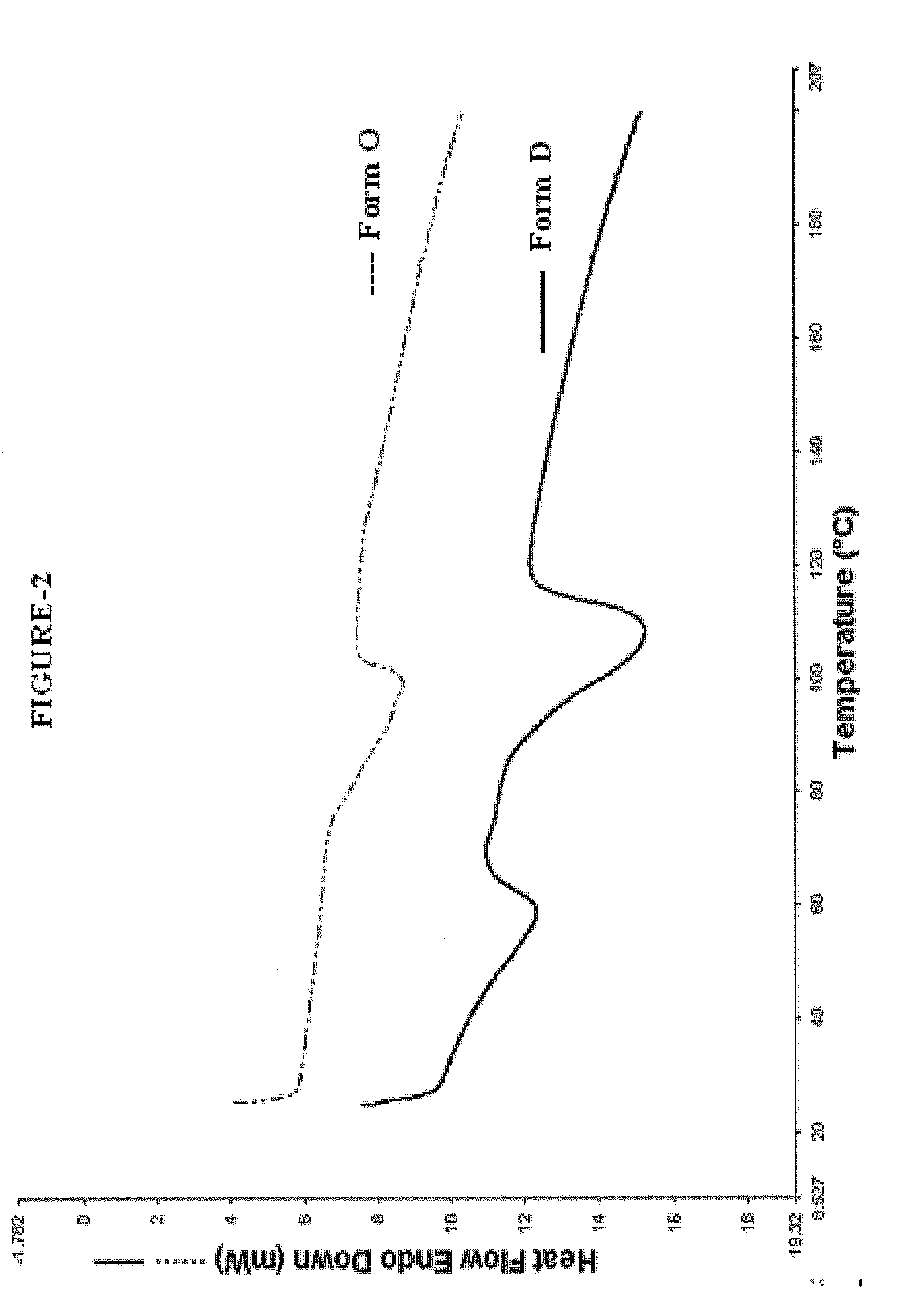
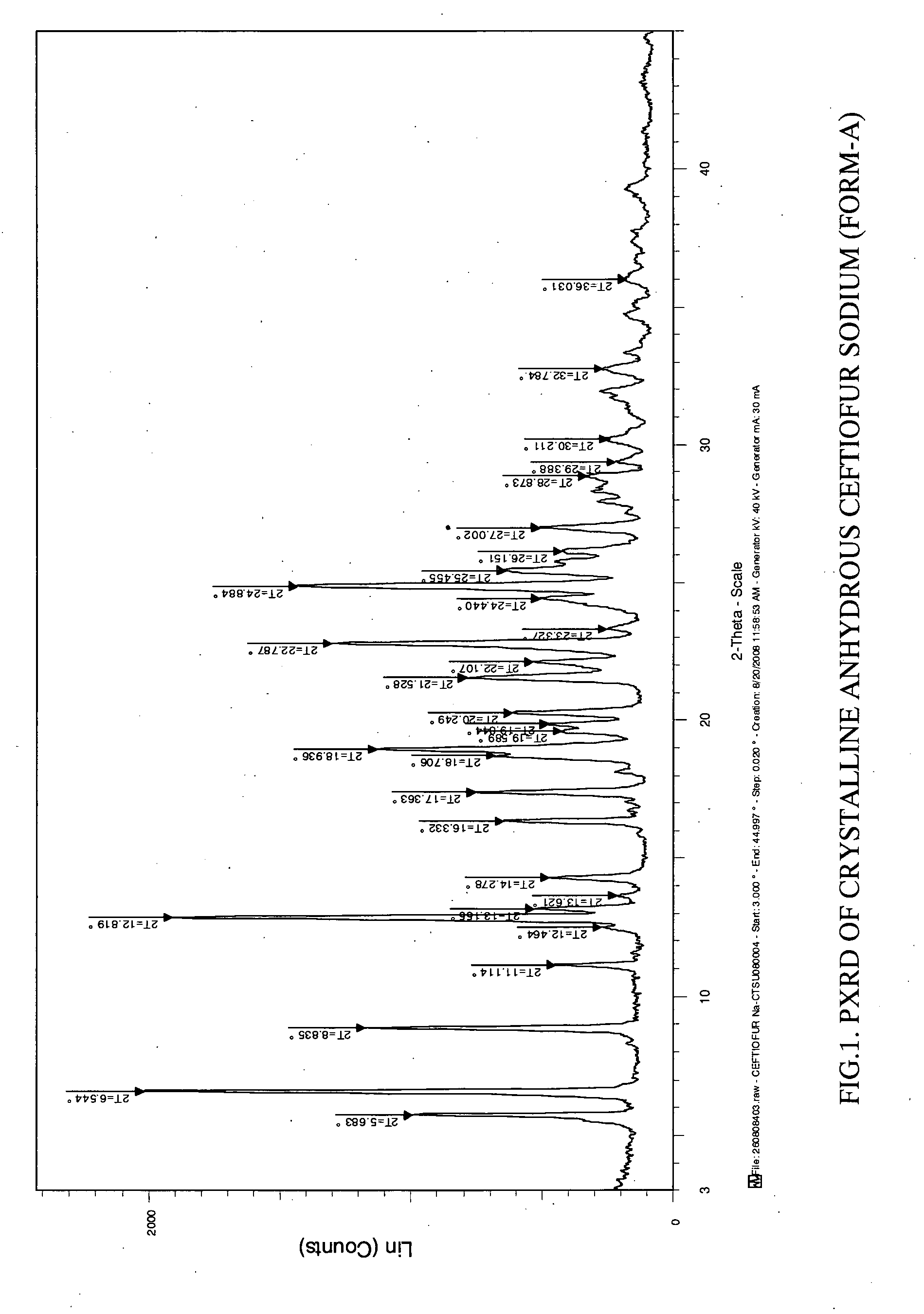
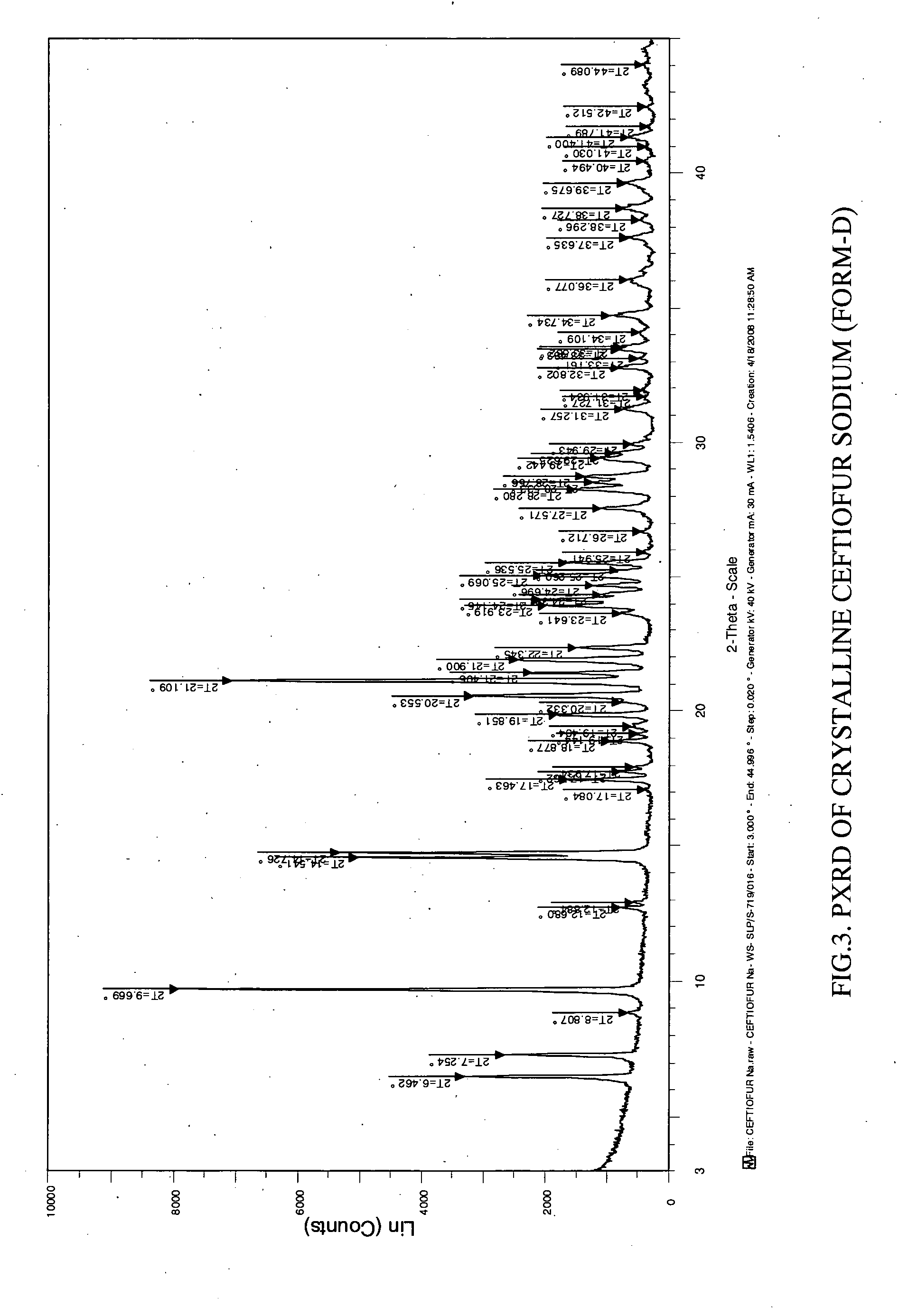
Crystalline sodium salt of cephalosporin antibiotic
ActiveUS20110059933A1Improve stabilityAntibacterial agentsOrganic active ingredientsCeftiofur sodiumCephalosporin Antibiotic
Owner:ORCHID CHEM & PHARM LTD
Preparation technology of ceftiofur sodium for injection, capable of preventing products from being unloaded
The invention relates to a preparation technology of ceftiofur sodium for injection, capable of preventing products from being unloaded. The preparation technology comprises the following steps of synthesizing the ceftiofur sodium: using 7- aminocephalosporanic acid as raw materials, performing a reaction so as to obtain triethanolamine salt of ceftiofur, and after the rriethanolamine salt reacts with sodium salt in methanol or tetrahydrofuran, obtaining precipitation of the ceftiofur sodium; drying prepared wet ceftiofur sodium products, and grinding the dried ceftiofur sodium products into powder. The preparation technology of the ceftiofur sodium comprises the following steps of firstly cleaning a reagent bottle for loading the ceftiofur sodium powder, then loading the ceftiofur sodium powder in the cleaned reagent bottle in a quantitative manner according to the specification, filling nitrogen in the reagent bottle, and then covering a pretreated rubber plug; performing cover pricking treatment by using an aluminum cover, so that products are sealed, and then sequentially pasting internal labels, packaging and lamp inspection on the reagent bottle after cover pricking treatment is performed; and then, pasting external labels on a packing box, and performing inspection and package. According to the preparation technology disclosed by the invention, the problems that in the preparation technology of the ceftiofur sodium, a preparation process cannot be monitored in real time, and the products cannot be effectively managed and controlled are solved.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
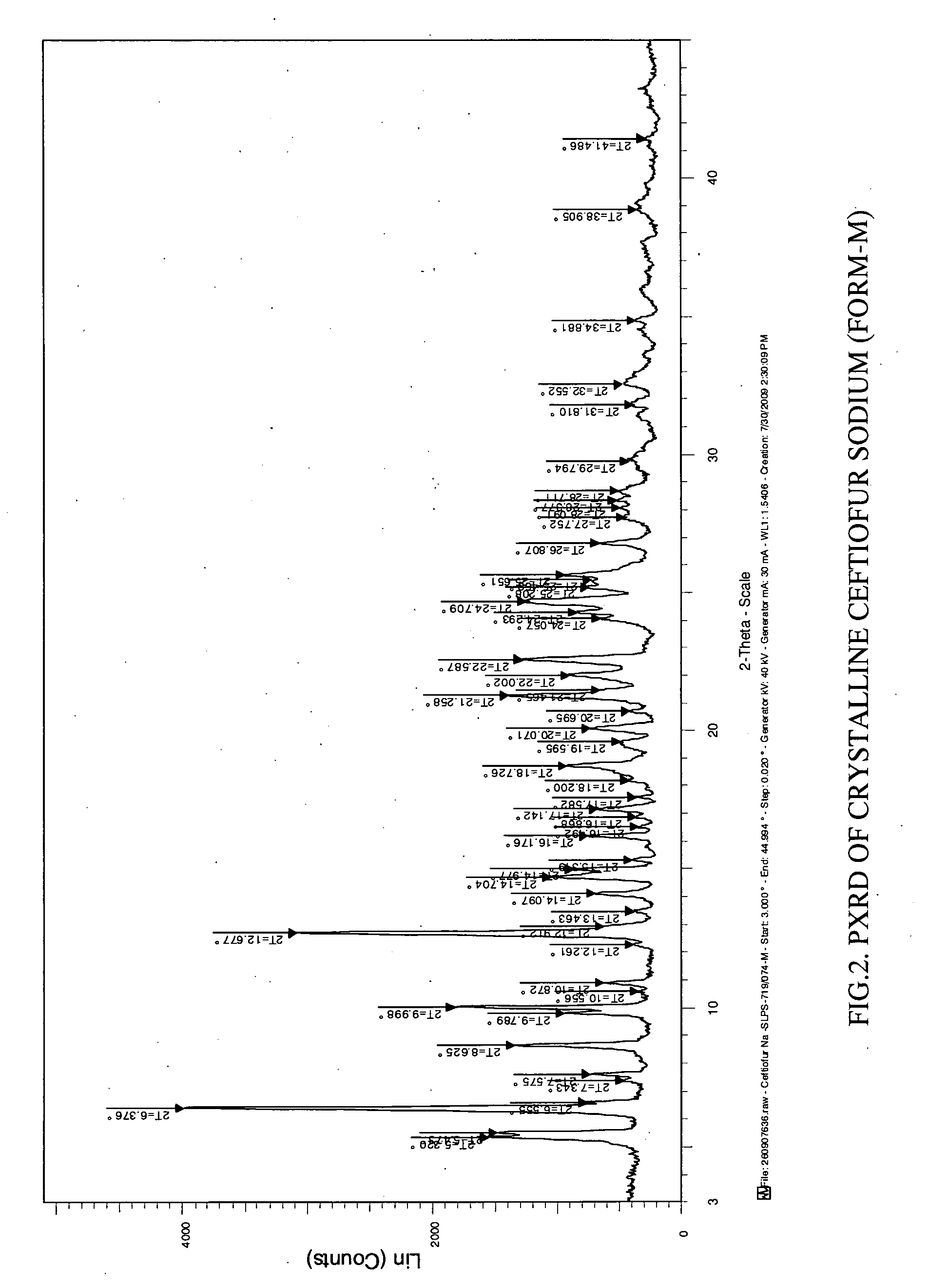
Crystalline sodium salt of cephalosporin antibiotic
ActiveUS20110136777A1Improve stabilityOrganic active ingredientsOrganic chemistryCeftiofur sodiumCephalosporin Antibiotic
Owner:ORCHID CHEM & PHARM LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com