Medicine formula for eliminating in-vivo enolotoxin
A bacterial endotoxin and drug technology, applied in antibacterial drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as endotoxin, poisoning death, fever, etc., to overcome drug resistance, high cure rate, and curative effect. significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
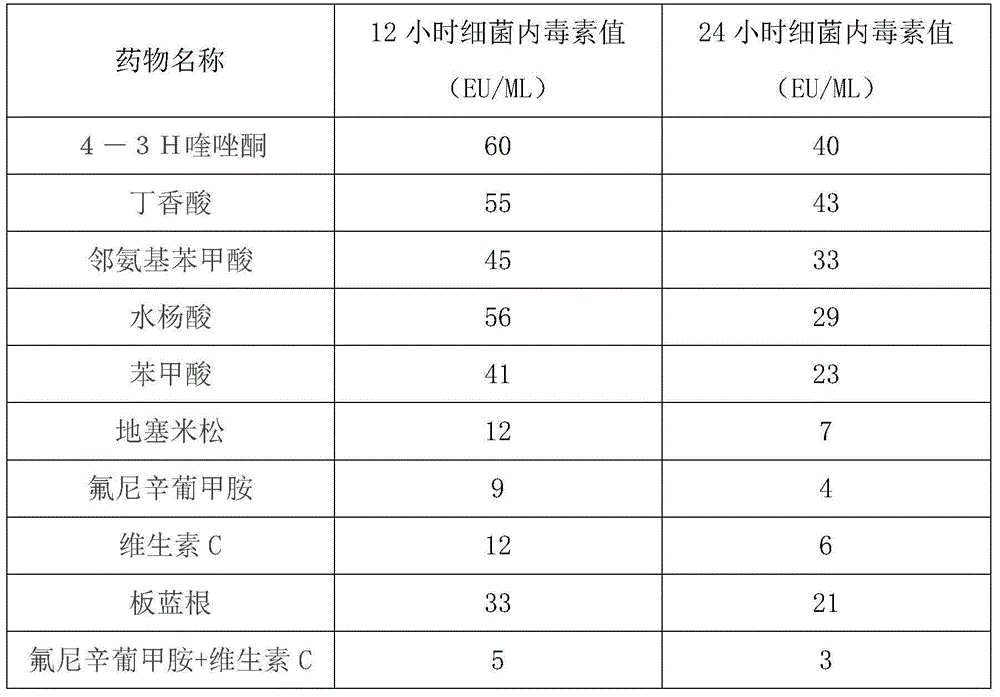
[0032] Formula A: flunixin meglumine 1mg / kg body weight, vitamin C 1mg / kg body weight;
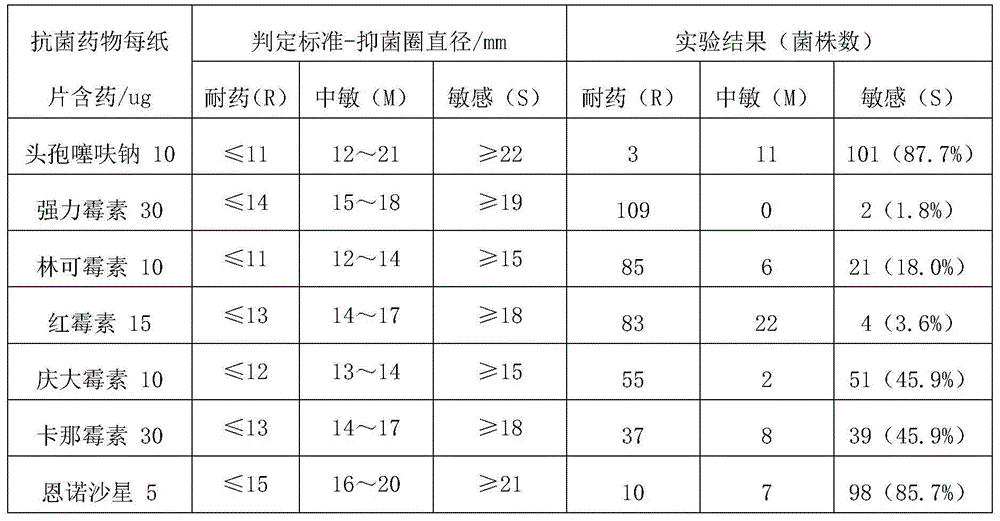
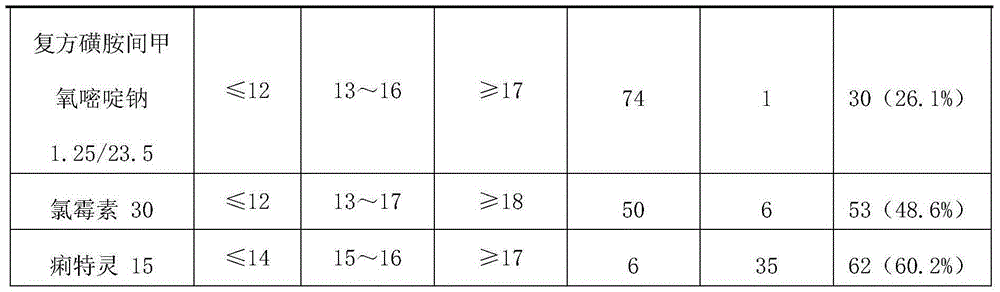
[0033] Formula B: ceftiofur sodium 2mg / kg body weight, enrofloxacin 1.5mg / kg body weight;
[0034] Described flunixin meglumine is the injection of 0.05g / ml, and described vitamin C is the injection of 0.1g / ml, and described enrofloxacin is the injection of 0.05g / ml, and described ceftiofur sodium is frozen Dry powder injection.
[0035] Formulas A and B were prepared respectively according to the above doses, and the formulas A and B were intramuscularly injected on both sides of the pig body respectively.
Embodiment 2
[0037] Formula A: flunixin meglumine 1.5mg / kg body weight, vitamin C 2mg / kg body weight;
[0038] Formula B: ceftiofur sodium 3mg / kg body weight, enrofloxacin 2mg / kg body weight;
[0039] Described flunixin meglumine is the injection of 0.05g / ml, and described vitamin C is the injection of 0.1g / ml, and described enrofloxacin is the injection of 0.05g / ml, and described ceftiofur sodium is frozen Dry powder injection.
[0040] Formulas A and B were prepared respectively according to the above doses, and the formulas A and B were intramuscularly injected on both sides of the pig body respectively.
Embodiment 3
[0042] Formula A: flunixin meglumine 2.5mg / kg body weight, vitamin C 3mg / kg body weight;
[0043] Formula B: ceftiofur sodium 5mg / kg body weight, enrofloxacin 2.5mg / kg body weight;
[0044] Described flunixin meglumine is the injection of 0.05g / ml, and described vitamin C is the injection of 0.1g / ml, and described enrofloxacin is the injection of 0.05g / ml, and described ceftiofur sodium is frozen Dry powder injection.
[0045] Formulas A and B were prepared respectively according to the above doses, and the formulas A and B were intramuscularly injected on both sides of the pig body respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com