Medicinal composition containing ceftiofur bisbenzylethylenediamine
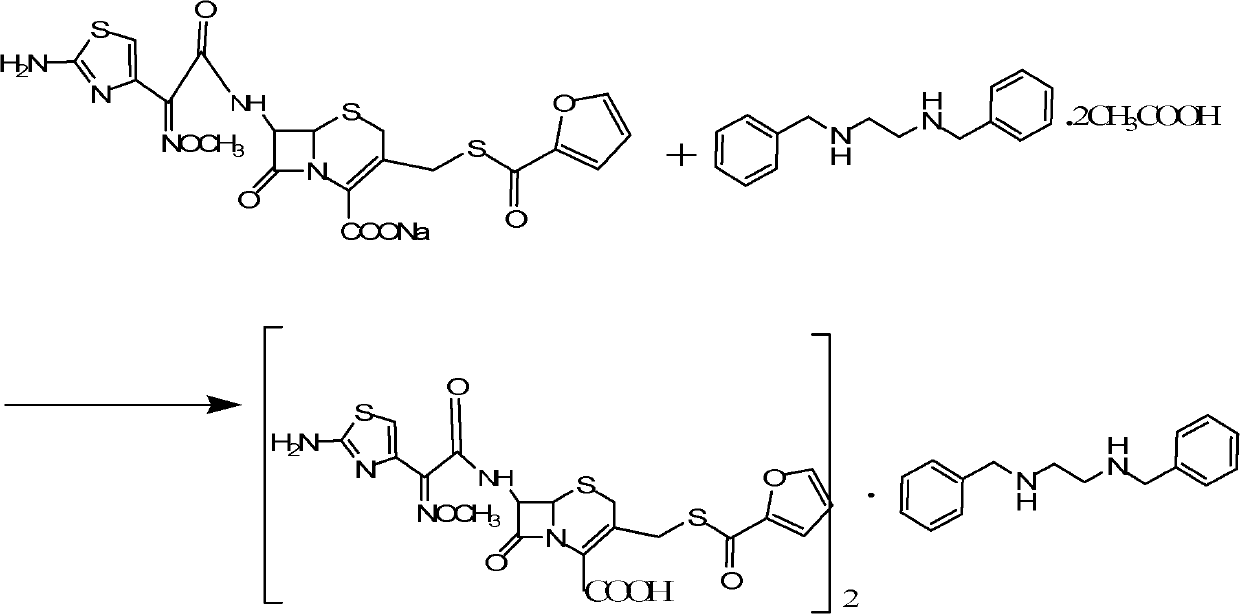
A technology for thiifurobisbenzylethylenediamine salt and cephalosporin, which is applied in the field of veterinary drug preparations, can solve the problems of not recognizing active ingredients and the like, and achieves the effects of long drug effect maintenance time, good drug effect and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
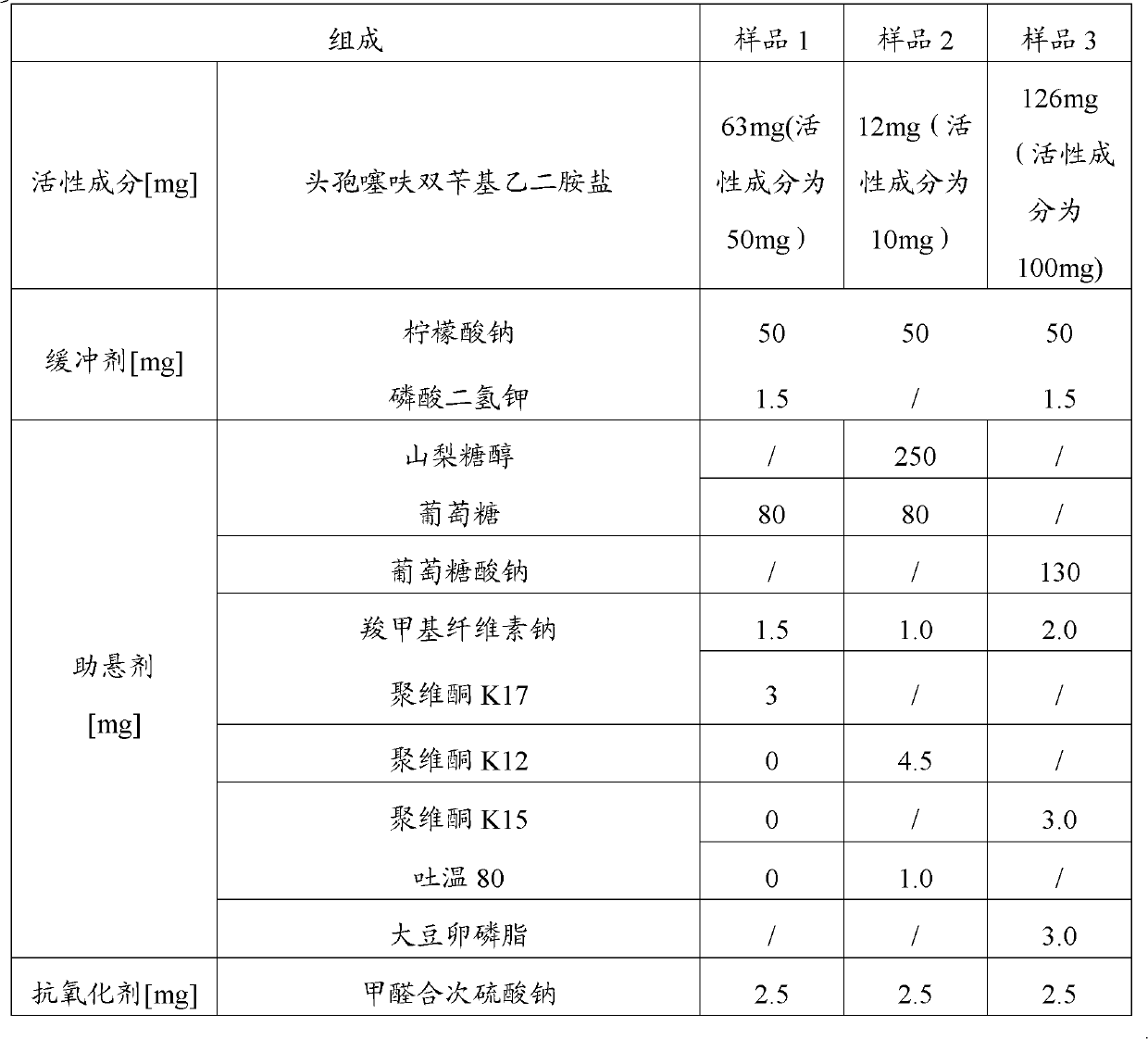
[0052] Prepare the suspension injection of ceftiofur bisbenzylethylenediamine salt according to the following components:
[0053]
[0054]
[0055] Wherein the ceftiofur bisbenzylethylenediamine salt is ultra-micronized, and the method is as described above.
[0056] According to the above formula, the method for preparing the ceftiofur bisbenzylethylenediamine salt suspension injection is as follows: the suspending agent is prepared into a solution according to the above ratio, and set aside. Dissolve the wetting agent, buffering agent and antioxidant in water according to the above ratio, and prepare solution a for future use. Add the suspension to liquid a to obtain liquid b. Adjust the pH to 7.0 ± 0.5 with sodium citrate solution. Add micronized ceftiofur bisbenzylethylenediamine salt, dilute to 1 mL with water for injection, and obtain three batches of samples—sample 1, 2 and 3.
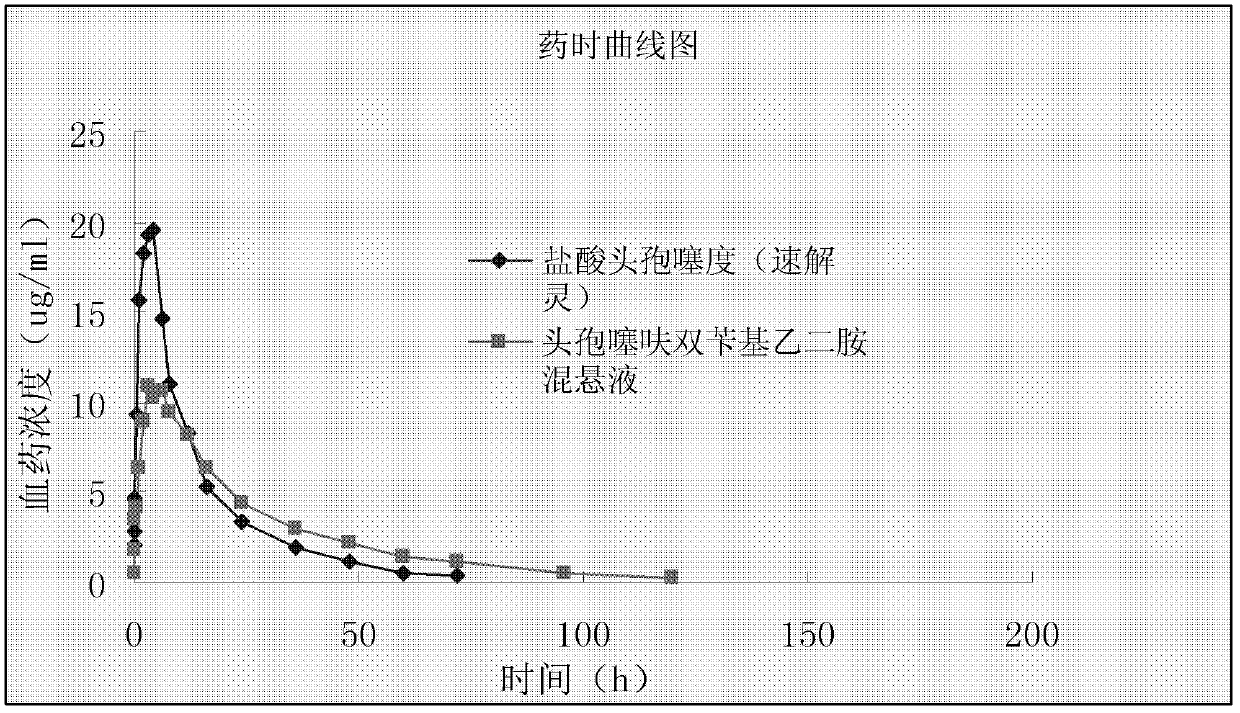
[0057] Drug efficacy, irritation and stability tests are carried out on sample 1 b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com