Preparation technology of ceftiofur sodium for injection, capable of preventing products from being unloaded
A technology of ceftiofur sodium and preparation process, applied in the field of medicine, can solve problems such as inability to control products, inability to monitor the preparation process, etc., and achieve the effects of less harsh reaction conditions, avoiding unlabeled, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
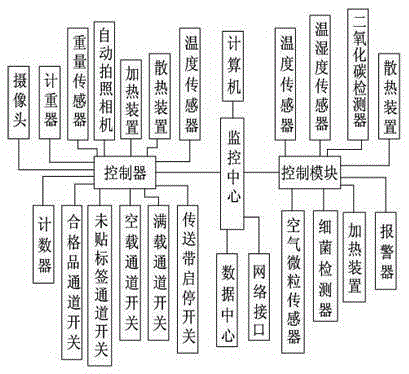
[0025] Such as figure 1 Shown, avoid the preparation technology of the ceftiofur sodium for injection of empty product, comprise the following steps:
[0026] 1) Synthesis of ceftiofur sodium: use 7-aminocephalosporanic acid as the raw material, use tetrahydrofuran and purified water as the solvent in a ratio of 1:3, and obtain the triethylamine salt of ceftiofur after reaction, and then in tetrahydrofuran After reacting with sodium isooctanoate, the precipitate of ceftiofur sodium is obtained, the ratio of tetrahydrofuran to sodium isooctanoate is 2:1, the precipitate is washed with ethanol, and the wet product of ceftiofur sodium is obtained after filtration under reduced pressure;
[0027] 2), drying and grinding the prepared ceftiofur sodium wet product into powder;
[0028] 3) The preparation process of ceftiofur sodium: first clean the reagent bottle used to hold the ceftiofur sodium powder, then quantitatively fill the ceftiofur sodium powder into the above cleaned rea...
Embodiment 2
[0031] Such as figure 1 Shown, avoid the preparation technology of the ceftiofur sodium for injection of empty product, comprise the following steps:
[0032] 1) Synthesis of ceftiofur sodium: 7-aminocephalosporanic acid is used as raw material, tetrahydrofuran and purified water are used as solvents in a ratio of 1:4, and the triethylamine salt of ceftiofur is obtained through reaction, and then in tetrahydrofuran After reacting with sodium isooctanoate, the precipitate of ceftiofur sodium is obtained, the ratio of tetrahydrofuran to sodium isooctanoate is 3:1, the precipitate is washed with n-propanol, and the wet product of ceftiofur sodium is obtained after filtration under reduced pressure;
[0033] 2), drying and grinding the prepared ceftiofur sodium wet product into powder;
[0034] 3) The preparation process of ceftiofur sodium: first clean the reagent bottle used to hold the ceftiofur sodium powder, then quantitatively fill the ceftiofur sodium powder into the above...
Embodiment 3
[0037] Such as figure 1 Shown, avoid the preparation technology of the ceftiofur sodium for injection of empty product, comprise the following steps:
[0038] 1) Synthesis of ceftiofur sodium: use 7-aminocephalosporanic acid as the raw material, use tetrahydrofuran and purified water as the solvent in a ratio of 1:5, react to obtain the triethylamine salt of ceftiofur, and then dissolve it in methanol After reacting with sodium 2-ethylhexanoate, the precipitate of ceftiofur sodium is obtained, the ratio of methanol to sodium isooctanoate is 2:1, the precipitate is washed with ethylene glycol, and then filtered under reduced pressure to obtain the wet product of ceftiofur sodium;
[0039] 2), drying and grinding the prepared ceftiofur sodium wet product into powder;
[0040] 3) The preparation process of ceftiofur sodium: first clean the reagent bottle used to hold the ceftiofur sodium powder, then quantitatively fill the ceftiofur sodium powder into the above cleaned reagent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com